NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Gabapentin enacarbil is a long acting form of gabapentin that is used for restless leg syndrome and for painful postherpetic neuropathy. Gabapentin enacarbil and gabapentin are associated with a low rate of transient serum enzyme elevations during treatment and with rare instances of clinically apparent liver injury.
Background
Gabapentin enacarbil (gab" a pen' tin) enacarbil (en" a kar' bil) is a prodrug of and long acting form of gabapentin. Gabapentin and its derivative gabapentin enacarbil do not appear to act upon gaba-ergic transmission, but appear to bind and perhaps inhibit presynaptic, voltage dependent calcium channels which play a role in normal and abnormal neurotransmission. In several prelicensure controlled trials, gabapentin enacarbil given in once daily doses was found to decrease symptoms of restless leg syndrome and postherpetic neuralgia. It has also been studied in other neuropathic pain syndromes, but with less obvious beneficial effects. Gabapentin enacarbil was approved for use in the United States in 2011 for the therapy of restless leg syndrome and postherpetic neuralgia. It is not approved as therapy for epilepsy, a major current indication for gabapentin itself. Gabapentin enacarbil is available in extended release tablets of 300 and 600 mg under the brand name Horizant. The typical dose is 600 to 1,200 mg once daily. Side effects may include somnolence, headache, dizziness, ataxia, blurred vision, nausea, fatigue and tremor.
Hepatotoxicity
In prelicensure clinical trials, gabapentin enacarbil was not linked to an increased rate of serum enzyme elevations during treatment or to episodes of clinically apparent acute liver injury. Gabapentin has been implicated in isolated cases of acute liver injury, but in most instances the association was weakened by the coadministration with other potentially hepatotoxic agents. Too few cases have been reported in sufficient detail to characterize the clinical features of the associated injury. No published case has specifically implicated gabapentin enacarbil. Thus, gabapentin enacarbil may be associated with isolated instances of clinically apparent liver injury, but they must be rare, if they occur at all.
Likelihood score: E* (unproven but suspected cause of liver injury).
Mechanism of Injury
Gabapentin enacarbil is hydrolyzed to gabapentin in the gastrointestinal tract. Gabapentin itself undergoes little subsequent metabolism and is excreted in the urine unchanged. Gabapentin and gabapentin enacarbil do not induce or inhibit the drug metabolizing, microsomal cytochrome P450 enzymes. The possible mechanism by which gabapentin might cause hepatic injury is not known, but may relate to a hypersensitivity reaction.
Outcome and Management
Gabapentin enacarbil is metabolized to gabapentin and its side effect profile is similar, suggesting that it should not be used in patients with severe adverse reactions to gabapenin. There is no information on the possible cross sensitivity to hepatotoxicity between gabapentin enacarbil and other anticonvulsants, but its structure would suggest that there should not be shared sensitivity.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Gabapentin Enacarbil – Horizant®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
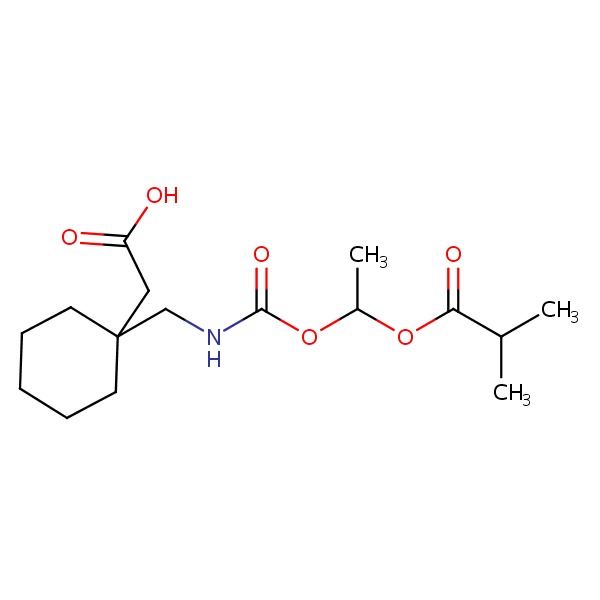
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Gabapentin Enacarbil | 478296-72-9 | C16-H27-N-O6 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 February 2018
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013 does not discuss either gabapentin or gabapentin enacarbil).
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999 before the availability of gabapentin enacarbil).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; gabapentin, but not its enacarbil is discussed).
- McNamara JO. Pharmacotherapy of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, p. 602.(Textbook of pharmacology and therapeutics).
- Galindo PA, Borja J, Gómez E, Mur P, Gudín M, García R, Encinas C, et al. Anticonvulsant drug hypersensitivity. J Investig Allergol Clin Immunol 2002; 12: 299-304. [PubMed: 12926190](Among 15 patients with cutaneous hypersensitivity reactions to anticonvulsants [9 accompanied by liver test elevations] which were caused by carbamazepine [n=8], phenytoin [5], lamotrigine [4], phenobarbital [4], valproate [1] and felbamate [1], one of whom later tolerated gabapentin without recurrence).
- Himmerich H, Nickel T, Dalal MA, Müller MB. [Gabapentin treatment in a female patient with panic disorder and adverse effects under carbamazepine during benzodiazepine withdrawal]. Psychiatr Prax 2007; 34: 93-4. German. [PubMed: 17124639](70 year old woman developed serum enzyme elevations on melperone and carbamazepine [ALT 103 U/L], but then tolerated gabapentin without recurrence).
- Kummer O, Hammann F, Bodmer M, Novakova K, Krähenbühl S, Haschke M. [Drug-induced toxic hepatitis]. Praxis (Bern 1994) 2008; 97: 235-9; German. [PubMed: 18548805](55 year old man developed jaundice 12 days after starting a Chinese herbal medication called Hong Hua and while receiving gabapentin, resolving within 3 months of stopping all medications).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scan 2008; 118: 281-90. [PubMed: 18341684](Review of all anticonvulsant induced liver injury; neither gabapentin nor pregabalin are discussed).
- Bogan RK, Bornemann MA, Kushida CA, Trân PV, Barrett RW; XP060 Study Group. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. Mayo Clin Proc 2010; 85: 512-21. [PMC free article: PMC2878254] [PubMed: 20511481](Among 194 patients treated with gabapentin enacarbil [1200 mg daily] or placebo for 12 weeks after receiving the active drug for at least 24 weeks, somnolence and dizziness were the most common adverse events and “there were no clinically relevant changes in laboratory values…for either phase or treatment group”).
- Gabapentin encarbil (Horizant) for restless leg syndrome. Med Lett Drugs Ther 2011; 53 (1372): 70-1. [PubMed: 21897349](Concise review of the pharmacology, clinical efficacy, adverse events and costs of gabapentin encarbil, shortly after its approval for restless leg syndrome in the US, mentions its common side effects of somnolence, dizziness, ataxia, edema, weight gain, blurred vision, disorientation, lethargy and vertigo, but does not mention ALT elevations or hepatotoxicity).
- Lee DO, Ziman RB, Perkins AT, Poceta JS, Walters AS, Barrett RW; XP053 Study Group. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med 2011; 7: 282-92. [PMC free article: PMC3113968] [PubMed: 21677899](Among 325 patients with restless leg syndrome treated with gabapentin enacarbil [600 or 1200 mg daily] or placebo for 12 weeks, common side effects included dizziness and somnolence and there were no serious hepatic adverse events; “no clinically relevant changes in…laboratory parameters were observed”).
- Ellenbogen AL, Thein SG, Winslow DH, Becker PM, Tolson JM, Lassauzet ML, Chen D. A 52-week study of gabapentin enacarbil in restless legs syndrome. Clin Neuropharmacol 2011; 34: 8-16. Med . [PubMed: 21242741](Among 573 patients with restless leg syndrome who participated in randomized controlled trials who were enrolled in a 52 week open label extension study of gabapentin enacarbil [600-1800 mg daily], 20 patients had a serious adverse reaction, but none were liver related and there were “no clinically relevant changes” in laboratory parameters, although five patients withdrew from therapy because of elevations in serum enzymes including ALT [peak values 75 to 154 U/L]).
- Lal R, Ellenbogen A, Chen D, Zomorodi K, Atluri H, Luo W, Tovera J, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol 2012; 35: 165-73. [PubMed: 22664749](Among 217 patients with restless leg syndrome treated with gabapentin enacarbil [600, 1200, 1800 or 2400 mg daily] or placebo for 12 weeks, drug levels and side effects were dose related, but there were no hepatic serious adverse events and “no clinically significant changes in…laboratory parameters were observed”).
- Hayes WJ, Lemon MD, Farver DK. Gabapentin enacarbil for treatment of restless legs syndrome in adults. Ann Pharmacother 2012; 46: 229-39. [PubMed: 22298601](Systematic review of the literature on use of gabapentin enacarbil for restless leg syndrome, mentions neuropsychiatric adverse events, but not hepatotoxicity or ALT elevations).
- Harden RN, Freeman R, Rainka M, Zhang L, Bell C, Berges A, Chen C, et al. A phase 2a, randomized, crossover trial of gabapentin enacarbil for the treatment of postherpetic neuralgia in gabapentin inadequate responders. Pain Med 2013; 14: 1918-32. [PubMed: 24102928](Among 94 patients with postherpetic neuralgia treated with 1200 or 3600 mg of gabapentin enacarbil daily, no patient had to stop therapy because of liver injury and there were no differences in laboratory tests between the two doses).
- Zhang L, Rainka M, Freeman R, Harden RN, Bell CF, Chen C, Graff O, et al. A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of gabapentin enacarbil in subjects with neuropathic pain associated with postherpetic neuralgia (PXN110748). J Pain 2013; 14: 590-603. [PubMed: 23602345](Among 371 patients with postherpetic neuralgia treated with 1 of 3 doses of gabapentin enacarbil or placebo for 14 weeks, “there were no noticeable differences among groups” in laboratory test results).
- Nagandla K, De S. Restless legs syndrome: pathophysiology and modern management. Postgrad Med J 2013; 89: 402-10. [PubMed: 23524988](Review of the clinical features, pathophysiology and treatment of restless leg syndrome; no discussion of side effects of most agents or mention of ALT elevations or hepatotoxicity).
- Rauck R, Makumi CW, Schwartz S, Graff O, Meno-Tetang G, Bell CF, Kavanagh ST, McClung CL. A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract 2013; 13: 485-96. [PubMed: 23186035](Among 421 adults with diabetic neuropathy treated with 1 of 3 doses of gabapentin enacarbil or pregabalin or placebo for 20 weeks, pain intensity scores did not change from baseline to a significant extent in any group, while side effects were greater with the drug treatments, although “there were no systematic changes noted in laboratory parameters”).
- Silberstein S, Goode-Sellers S, Twomey C, Saiers J, Ascher J. Randomized, double-blind, placebo-controlled, phase II trial of gabapentin enacarbil for migraine prophylaxis. Cephalalgia 2013; 33: 101-11. [PubMed: 23165696](Among 523 patients with migraine treated with gabapentin enacarbil [1200-300 mg daily] or placebo for 20 weeks, there was no decrease in frequency of migraine headaches with treatment, no hepatic serious adverse events, and “no significant changes in laboratory values”).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; gabapentin is approved for use in partial seizures and neuropathic pain and its prodrug, gabapentin enacarbil, is approved for restless leg syndrome, but not for epilepsy).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96, none were attributed to gabapentin enacarbil).
- Fuzier R, Serres I, Guitton E, Lapeyre-Mestre M, Montastruc JL; French Network of Pharmacovigilance Centres. Adverse drug reactions to gabapentin and pregabalin: a review of the French pharmacovigilance database. Drug Saf 2013; 36: 55-62. [PubMed: 23315296](Among 725 spontaneous adverse event reports related to gabapentin made to the French Pharmacovigilance System between 1995 and 2009, liver ranked second to neuropsychiatric reactions in frequency [n=90, 12%], 37 of which were “hepatitis”, half of which were serious, 8 were “probable” or “likely” and one fatal, but no specific details given).
- Gabapentin and pregabalin: hepatic and haematological toxicity. Prescrire Int 2014; 23 (154): 267. [PubMed: 25954794](Review of spontaneous reports of adverse events attributed to gabapentin from a French registry [Fuzier 2013] identified 90 cases of liver damage, gabapentin being the only suspect drug in 10 cases, one of which was fatal).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, including 3 to gabapentin, but none specifically to gabapentin enacarbil).
- Zaccara G, Giovannelli F, Giorgi FS, Franco V, Gasparini S, Benedetto U. Tolerability of new antiepileptic drugs: a network meta-analysis. Eur J Clin Pharmacol 2017; 73: 811-7. [PubMed: 28378057](Metanalysis of the comparative tolerability of 18 new anticonvulsant agents including gabapentin, found lowest rates of withdrawal for adverse events with levetiracetam, brivaracetam, and gabapentin with highest rates with eslicarbazepine, oxcarbazepine, lacosamide and topiramate).
- Drugs for epilepsy. Med Lett Drugs Ther 2017; 59 (1526): 121-30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists gabapentin enacarbil as a form of gabapentin approved for use in restless leg syndrome and mentions common side effects of gabapentin being somnolence, dizziness, ataxia, fatigue, blurred vision and confusion; no mention of ALT elevations or hepatotoxicity).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic Drugs and Liver Disease. Pediatr Neurol 2017; 77: 23-36. [PubMed: 29097018](Summary of the hepatotoxicity of major anticonvulsant medications including gabapentin which has no hepatic metabolism, so specific recommendation for dose modification because of liver disease, a minimal potential for drug-interations and low assocation with hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Gabapentin enacarbil: in patients with restless legs syndrome.[CNS Drugs. 2012]Review Gabapentin enacarbil: in patients with restless legs syndrome.Scott LJ. CNS Drugs. 2012 Dec; 26(12):1073-83.
- Review Gabapentin enacarbil in restless legs syndrome.[Drugs Today (Barc). 2010]Review Gabapentin enacarbil in restless legs syndrome.Merlino G, Serafini A, Lorenzut S, Sommaro M, Gigli GL, Valente M. Drugs Today (Barc). 2010 Jan; 46(1):3-11.
- Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome.[Mov Disord. 2011]Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome.Winkelman JW, Bogan RK, Schmidt MH, Hudson JD, DeRossett SE, Hill-Zabala CE. Mov Disord. 2011 Sep; 26(11):2065-72. Epub 2011 May 24.
- Review of the treatment of restless legs syndrome: focus on gabapentin enacarbil.[J Cent Nerv Syst Dis. 2012]Review of the treatment of restless legs syndrome: focus on gabapentin enacarbil.Burke RA, Faulkner MA. J Cent Nerv Syst Dis. 2012; 4:147-56. Epub 2012 Sep 17.
- Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study.[Mayo Clin Proc. 2010]Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study.Bogan RK, Bornemann MA, Kushida CA, Trân PV, Barrett RW, XP060 Study Group. Mayo Clin Proc. 2010 Jun; 85(6):512-21.
- Gabapentin Enacarbil - LiverToxGabapentin Enacarbil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...