NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Fosphenytoin is a prodrug of phenytoin available in parenteral forms only. While not specifically associated with cases of drug induced liver injury, fosphenytoin is converted to phenytoin which is a well known cause of acute idiosyncratic drug induced liver disease.
Background
Fosphenytoin (fos' fen i toyn") is a hydantoin derivative that is a prodrug of phenytoin that is rapidly converted to phenytoin and is likely to have the same mechanisms of activity, efficacy and side effects as phenytoin. Phenytoin acts by stabilization of neuronal membranes through increasing the efflux and decreasing the influx of sodium ions across GABA regulated Na+ channels in neuron cell membranes. Fosphenytoin was approved for use in the United States in 1996. Current indications are for the short term treatment of tonic-clonic seizures and status epilepticus, for prevention and treatment of seizures during neurosurgery and for temporary replacement of phenytoin when oral administration is not possible. Fosphenytoin is available in parenteral formulations in 2 and 10 mL vials in concentrations of 50 mg/mL generically and under the brand name Cerebyx. The recommended dose in adults is a loading dose of 10 to 20 phenytoin sodium equivalents per kilogram intravenously initially, and then a maintenance dose of 4 to 6 equivalents per kilogram each day in divided doses. Once oral therapy can be introduced, phenytoin is usually substituted. Common adverse events include pruritus, nystagmus, dizziness, somnolence, nausea and vomiting. Rare but potentially severe adverse reactions include cardiac arrhythmias, hypotension and bradycardia with rapid infusions for which reason the infusion rate should not exceed 50 phenytoin mg equivalents per minute in adults and 2 equivalents per kilogram per minute in children. Other uncommon severe adverse reactions include hypersensitivity reactions, angioedema, DRESS and Stevens Johnson syndrome.
Hepatotoxicity
Hepatic injury has not been specifically ascribed to use of fosphenytoin, but because it is metabolized to phenytoin, it is likely to cause similar hepatic injury. Cases of typical immunoallergic hepatitis and DRESS syndrome have been reported in patients initially treated with fosphenytoin and then converted to the oral form.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of fosphenytoin and phenytoin hepatotoxicity is not known, but is thought to be immunoallergic.
Outcome and Management
The course and outcome of hepatotoxicity from fosphenytoin should be similar to that of phenytoin.
References on the safety and hepatotoxicity of fosphenytoin are included in the Annotated Bibliography of Phenytoin, last updated in July 2020.
Drug Class: Anticonvulsants; see also Phenytoin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Fosphenytoin – Generic, Cerebyx®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
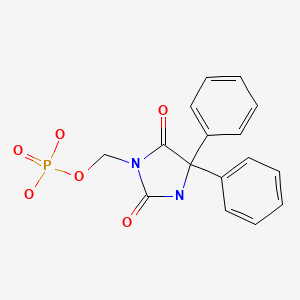
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Fosphenytoin | 93390-81-9 | C16-H15-N2-O6-P |
|
- PubChem SubstanceRelated PubChem Substances
- Review Fosphenytoin.[Neurol Res. 1998]Review Fosphenytoin.Luer MS. Neurol Res. 1998 Mar; 20(2):178-82.
- Review Fosphenytoin: a novel phenytoin prodrug.[Pharmacotherapy. 1996]Review Fosphenytoin: a novel phenytoin prodrug.Boucher BA. Pharmacotherapy. 1996 Sep-Oct; 16(5):777-91.
- The safety and efficacy of fosphenytoin for the treatment of status epilepticus.[Expert Rev Neurother. 2015]The safety and efficacy of fosphenytoin for the treatment of status epilepticus.Popławska M, Borowicz KK, Czuczwar SJ. Expert Rev Neurother. 2015; 15(9):983-92. Epub 2015 Aug 9.
- Review Clinical experience with fosphenytoin in adults: pharmacokinetics, safety, and efficacy.[J Child Neurol. 1998]Review Clinical experience with fosphenytoin in adults: pharmacokinetics, safety, and efficacy.Knapp LE, Kugler AR. J Child Neurol. 1998 Oct; 13 Suppl 1:S15-8; discussion S30-2.
- Fosphenytoin: current place in therapy.[J Pediatr Pharmacol Ther. 2004]Fosphenytoin: current place in therapy.Mueller EW, Boucher BA. J Pediatr Pharmacol Ther. 2004 Oct; 9(4):265-73.
- Fosphenytoin - LiverToxFosphenytoin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...