NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Fexofenadine is a second generation antihistamine that is used for the treatment of allergic rhinitis, angioedema and chronic urticaria. Fexofenadine has not been linked to serum enzyme elevations during therapy or to instances of clinically apparent acute liver injury.
Background
Fexofenadine (fex" oh fen' a deen) is a second generation antihistamine (H1 receptor blocker) that is used widely to treat allergic symptoms associated with hay fever, seasonal allergies, urticaria, angioedema and atopic dermatitis. Fexofenadine, like other second generation antihistamines, is considered to be nonsedating, and prospective studies have shown that sedation is less common with it than with first generation antihistamines such as diphenhydramine. Fexofenadine was approved for use by prescription in the United States in 1996 and as an over-the-counter medication in 2011. Fexofenadine is one of the most widely used medications with more than 18 million prescriptions filled yearly in addition to considerable nonprescription use. Fexofenadine is available in 30, 60 and 180 mg tablets and capsules in generic forms and under the trade name Allegra. Oral suspensions, extended release tablets, and combinations with pseudoephrine are also available. The typical dose in adults is 30 to 60 mg twice daily or 180 mg once daily. Fexofenadine is often given chronically, at least during allergic season. Common side effects include blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Although considered to be a nonsedating antihistamine, fexofenadine can cause mild drowsiness particularly at higher doses. Antihistamines can worsen urinary retention and glaucoma.
Hepatotoxicity
Fexofenadine use is not generally associated with liver enzyme elevations but terfenadine, a second generation antihistamine that is metabolized in part to fexofenadine, was the attributed cause of several reported cases of clinically apparent liver injury. Terfenadine was also linked to cardiac arrhythmias and prolongation of the QTc interval for which reason it was withdrawn from use in 1997, just as fexofenadine was introduced. Liver injury from terfenadine typically arose after 1 to 5 months and was associated with a hepatocellular pattern of liver enzyme abnormalities without immunoallergic or autoimmune features. Fexofenadine has not been linked to similar cases of cardiac arrhythmias or liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
References on the safety and potential hepatotoxicity of antihistamines are given together after the Overview section on Antihistamines.
Drug Class: Antihistamines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Fexofenadine – Generic, Allegra®
DRUG CLASS
Antihistamines
Product labeling at DailyMed, National Library of Medicine, NIH
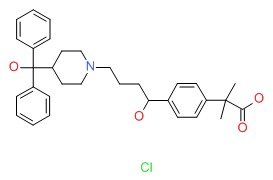
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Fexofenadine | 153439-40-8 | C32-H39-N-O4.Cl-H |
 |
- Fexofenadine: a review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria.[Drugs. 2000]Fexofenadine: a review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria.Simpson K, Jarvis B. Drugs. 2000 Feb; 59(2):301-21.
- Fexofenadine: new preparation. Terfenadine, without cardiotoxicity.[Prescrire Int. 1999]Fexofenadine: new preparation. Terfenadine, without cardiotoxicity.. Prescrire Int. 1999 Feb; 8(39):11-3.
- Reduction of side-effects from ultrarush immunotherapy with honeybee venom by pretreatment with fexofenadine: a double-blind, placebo-controlled trial.[Allergy. 2000]Reduction of side-effects from ultrarush immunotherapy with honeybee venom by pretreatment with fexofenadine: a double-blind, placebo-controlled trial.Reimers A, Hari Y, Müller U. Allergy. 2000 May; 55(5):484-8.
- Review Treatment of allergic rhinitis and urticaria: a review of the newest antihistamine drug bilastine.[Ther Clin Risk Manag. 2016]Review Treatment of allergic rhinitis and urticaria: a review of the newest antihistamine drug bilastine.Wang XY, Lim-Jurado M, Prepageran N, Tantilipikorn P, Wang de Y. Ther Clin Risk Manag. 2016; 12:585-97. Epub 2016 Apr 13.
- Review A review of the efficacy of desloratadine, fexofenadine, and levocetirizine in the treatment of nasal congestion in patients with allergic rhinitis.[Clin Ther. 2009]Review A review of the efficacy of desloratadine, fexofenadine, and levocetirizine in the treatment of nasal congestion in patients with allergic rhinitis.Bachert C. Clin Ther. 2009 May; 31(5):921-44.
- Fexofenadine - LiverToxFexofenadine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...