NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Eugenol, also called clove oil, is an aromatic oil extracted from cloves that is used widely as a flavoring for foods and teas and as an herbal oil used topically to treat toothache and more rarely to be taken orally to treat gastrointestinal and respiratory complaints. Eugenol in therapeutic doses has not been implicated in causing serum enzyme elevations or clinically apparent liver injury, but ingestions of high doses, as with an overdose, can cause severe liver injury.
Background
Eugenol (ue gen’ ol) is the major constituent [70% to 90%] in the aromatic oil extract from cloves (Syzygium aromaticum), a spice widely used as a flavoring for meats, stews, cakes and teas. Eugenol is also found in lower concentrations in cinnamon and other aromatic spices. The clove is native to the Moluccas, a chain of small islands that are now part of Indonesia and were formerly known as the Spice Islands. Cloves are now grown in several tropical regions and the spice sold as intact flower buds or as a ground powder. Eugenol is the most abundant ingredient in clove oil and is thought to be responsible for its aromatic as well as both beneficial and harmful effects. In vitro, eugenol has been shown to have antibacterial, antifungal, antioxidant and antineoplastic activity. Clove oils including eugenol have been claimed to have gentle local anesthetic and antiseptic activities and previously were commonly used in dentistry. Eugenol and clove extracts have also been purposed to be beneficial for gastrointestinal complaints such as nausea, diarrhea, abdominal pain and for cough, phlegm and chest congestion (as an expectorant). However, there are no convincing data to support the efficacy for any of these conditions, and neither eugenol or other clove extracts have been approved for use in any medical condition in the United States. Nevertheless, clove extracts are found in many topical creams, lotions and bath oils and occasionally in toothpaste for its possible effect in alleviating toothache or painful gums and in electronic cigarette refills. Vials of clove oil are available in health food and ethnic grocery stores and on the internet. Clove oil is advertised as having powerful antioxidant benefits and being useful for toothache, cleaning teeth and freshening of the breath. Clove cigarettes consisting of 60% to 70% tobacco and 30% to 40% cloves were imported from Indonesia and became popular in the United States in the early 1980’s when they were ultimately linked to a dozen cases of severe lung injury and banned from sale in many states. In addition, eugenol in higher doses has been used as an “organic” insecticide in households and gardens. Thus, eugenol used in low doses appears to have few side effects other than local irritation, rare allergic reactions and contact dermatitis, while exposure or ingestion of a large amounts, as in overdose, can result in tissue injury and a syndrome of acute onset of seizures, coma and damage to the liver and kidneys.
Hepatotoxicity
The low concentrations of eugenol and clove extracts used topically and in herbal products have not been convincingly linked to instances of liver injury, either in the form of serum enzyme elevations or clinically apparent liver injury. In high doses, however, eugenol appears to be a direct cytotoxin and several instances of severe acute liver and kidney injury have been reported after accidental overdose of eugenol containing herbal products, largely in children. Overdoses have been marked by the onset of agitation, decrease in consciousness and coma arising within hours on ingestion (10-30 mL of clove oil). There is typically an accompanying acidosis, respiratory depression and severe hypoglycemia requiring ventilation and intravenous glucose. Liver injury arises 12 to 24 hours after ingestion with marked elevations in serum aminotransferase levels and early coagulation abnormalities. Signs of hepatic failure arise rapidly, and jaundice can develop and deepen. The overall clinical presentation is typical of acute hepatic necrosis and similar to that of acetaminophen, iron or copper overdose. The liver injury generally worsens for several days but then rapidly improves and ultimately resolves within 1 to 3 weeks. Renal dysfunction may also occur but rarely requires intervention or dialysis. Long term injury or effects have not been described. Cases described in the literature have been in infants who swallowed clove oil being used by parents.
Likelihood score: C[H] (probable cause of clinically apparent liver injury in overdoses).
Mechanism of Injury
In high concentrations, eugenol has cytotoxic activity both in vitro and in vivo, characteristics [along with its aromatic qualities] that support its use as an insecticide. Thus, liver injury from eugenol is likely direct hepatotoxicity.
Outcome and Management
The acute liver injury of eugenol overdose presents clinically with acute hepatic necrosis, a pattern similar to that of acetaminophen hepatotoxicity. For these reasons, N-acetyl cysteamine has been used to treat patients with eugenol or clove oil overdose. While not proven to be effective for eugenol overdose in humans, it has been shown to be effective in preventing hepatic injury in animal models. Also important in management is careful attention to details of medical management including prevention of hypoglycemia and maintenance of electrolyte balance and respiratory support. If patients present early, use of activated charcoal or emetics may be useful.
Other Names: Clove oil
Drug Class: Herbal and Dietary Supplements
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Eugenol – Generic (OTC Products)
DRUG CLASS
Herbal and Dietary Supplements
Fact Sheet at MedlinePlus, NLM [Clove Oil]
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
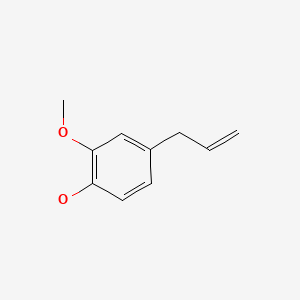
| Eugenol | 97-53-0 | C10-H12-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 15 October 2019
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 731-4.(Expert review of hepatotoxicity published in 1999; several herbals are discussed, including comfrey, germander, chaparral leaf, skullcap and valerian, but not eugenol or clove oil).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbal and dietary supplements [HDS]; clove oil is not discussed).
- Clove. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc., 2007: pp. 201-4.(Compilation of short monographs on herbal medications and dietary supplements).
- Barkin ME, Boyd JP, Cohen S. Acute allergic reaction to eugenol. Oral Surg Oral Med Oral Pathol. 1984;57:441–2. [PubMed: 6584843](Case report of allergic reaction to a temporary zinc oxide-eugenol dental restoration that required corticosteroid therapy and recurred after exposure to a similar dental sealer).
- Centers for Disease Control (CDC). Illnesses possibly associated with smoking clove cigarettes. MMWR Morb Mortal Wkly Rep. 1985;34:297–9. [PubMed: 3923308](Initial report of 2 cases of sudden onset of severe lung injury in previously healthy California teenagers shortly after smoking clove cigarettes, initially thought to be bacterial pneumonia but not responding to antibiotics while reversing rapidly with corticosteroid therapy).
- Evaluation of the health hazard of clove cigarettes. Council on Scientific Affairs. JAMA. 1988;260:3641–4. [PubMed: 3057254](Report from an expert committee of the American Medical Association summarizes the chemistry, metabolism, toxicity and clinical evidence of acute pulmonary toxicity from clove cigarettes based upon review of 10 cases from the literature; 8 males, 2 females, ages 11 to 29, 7 from California, with history of smoking clove cigarettes within hours of presentation with severe lung injury, with two fatalities).
- American Academy of Pediatrics Committee on Substance Abuse. Hazards of clove cigarettes. Pediatrics. 1991;88:395–6. [PubMed: 1861948](Brief statement from a committee on substance abuse of American Academy of Pediatrics on the potential of clove cigarettes to cause severe liver injury).
- Thompson DC, Constantin-Teodosiu D, Moldéus P. Metabolism and cytotoxicity of eugenol in isolated rat hepatocytes. Chem Biol Interact. 1991;77:137–47. [PubMed: 1991333](Exposure of rat hepatocytes to eugenol caused glutathione depletion and cell death within hours, effects that were dose-dependent and were prevented by N-acetylcysteine).
- Mizutani T, Satoh K, Nomura H, Nakanishi K. Hepatotoxicity of eugenol in mice depleted of glutathione by treatment with DL-buthionine sulfoximine. Res Commun Chem Pathol Pharmacol. 1991;71:219–30. [PubMed: 2047567](Mice treated with eugenol developed hepatotoxicity only when pretreated with inhibitors of glutathione synthesis).
- Brown SA, Biggerstaff J, Savidge GF. Disseminated intravascular coagulation and hepatocellular necrosis due to clove oil. Blood Coagul Fibrinolysis. 1992;3:665–8. [PubMed: 1450336](2 year old boy ingested 10 mL of clove oil and rapidly developed stupor, seizures, hypoglycemia, acidosis and coagulopathy with liver injury arising a day later [bilirubin 2.4 rising to 4.0 mg/dL, ALT 1549 to 5293 U/L, Alk P 418 to 587 U/L], with hepatic encephalopathy and renal dysfunction ultimately resolving within the next 3 weeks).
- Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69:392–3. [PMC free article: PMC1029532] [PubMed: 8215554](2 year old boy with acute liver failure after ingestion of clove oil [peak INR 6.51] treated with multiple coagulation factors; probably the same case as described by Brown et al [1992]).
- Sarrami N, Pemberton MN, Thornhill MH, Theaker ED. Adverse reactions associated with the use of eugenol in dentistry. Br Dent J. 2002;193:257–9. [PubMed: 12353045](Two cases of painful gums and oral ulcers arising days to weeks after application of eugenol impregnated temporary dental dressings, resolving after removal; both patients later having positive skin-patch tests to eugenol).
- Eisen JS, Koren G, Juurlink DN, Ng VL. N-acetylcysteine for the treatment of clove oil-induced fulminant hepatic failure. J Toxicol Clin Toxicol. 2004;42:89–92. [PubMed: 15083943](3 month old girl ingested 8 mL of clove oil and rapidly developed obtundation followed the next day by rises in ALT [peak 8761 U/L], AST [peak >10,000 U/L] and INR [peak 3.86], accompanied by acidosis and hypoglycemia, treated with fresh frozen plasma and N-acetylcysteine with recovery over the next few days).
- Janes SE, Price CS, Thomas D. Essential oil poisoning: N-acetylcysteine for eugenol-induced hepatic failure and analysis of a national database. Eur J Pediatr. 2005;164:520–2. [PubMed: 15895251](15 month old boy developed agitation 1 hour after ingesting 10-20 mL of clove oil requiring ventilation and followed 15 hours later by marked rises in ALT [peak ~13,000 U/L] and INR [peak 2.0] with renal dysfunction [peak creatinine 11.8/xx] and acidosis [pH nadir 7.33], treated with vitamin K and N-acetylcysteine with resolution over the next 8 days).
- Deshpande A, Verma S, Macwan C. Allergic reaction associated with the use of eugenol containing dental cement in a young child. Austin Journal of Dentistry. 2014;1:1007.(6 year old girl developed pain and allergic rash around a temporary dental restoration using zinc oxide-eugenol cement, resolving on removal).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver toxicity attributed to HDS products, does not list or mention clove oil or eugenol).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107 (Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, does not list or mention clove oil or eugenol).
- Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, et al. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–15. [PMC free article: PMC6023725] [PubMed: 29903732](Various flavoring compounds including eugenol decrease nitric oxide production by venous endothelial cells and induce cell death in dose dependent manner).
- Behar RZ, Luo W, McWhirter KJ, Pankow JF, Talbot P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep. 2018;8:8288. [PMC free article: PMC5974410] [PubMed: 29844439](Chemical analysis of 39 commercial e-cigarette refill cartridges found 12 flavoring chemicals in concentrations above 1 mg/mL, eugenol being found in 6 [15%] in concentrations of 0.6 to 6 mg/mL).
- Bui TNPT, Mose KF, Andersen F. Eugenol allergy mimicking burning mouth syndrome. Contact Dermatitis. 2019;80:54–5. [PubMed: 30238469](68 year old woman with burning mouth syndrome for 2 years and positive patch test to eugenol mentioned that she used a eugenol mouthwash and chewed cloves daily, her symptoms improving with discontinuation of oral use of eugenol and cloves).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Toxicological assessment of kretek cigarettes Part 5: mechanistic investigations, inhalation toxicity.[Regul Toxicol Pharmacol. 2014]Toxicological assessment of kretek cigarettes Part 5: mechanistic investigations, inhalation toxicity.Roemer E, Dempsey R, Van Overveld FJ, Berges A, Pype J, Weiler H, Vanscheeuwijck P, Schorp MK. Regul Toxicol Pharmacol. 2014 Dec; 70 Suppl 1:S54-65. Epub 2014 Oct 22.
- Toxicological assessment of kretek cigarettes Part 4: mechanistic investigations, smoke chemistry and in vitro toxicity.[Regul Toxicol Pharmacol. 2014]Toxicological assessment of kretek cigarettes Part 4: mechanistic investigations, smoke chemistry and in vitro toxicity.Roemer E, Dempsey R, Lawless-Pyne J, Lukman S, Evans AD, Trelles-Sticken E, Wittke S, Schorp MK. Regul Toxicol Pharmacol. 2014 Dec; 70 Suppl 1:S41-53. Epub 2014 Oct 22.
- Appetite-enhancing effects of inhaling cinnamon, clove, and fennel essential oils containing phenylpropanoid analogues.[J Nat Med. 2020]Appetite-enhancing effects of inhaling cinnamon, clove, and fennel essential oils containing phenylpropanoid analogues.Ogawa K, Honda M, Tanigawa A, Hatase A, Ito A, Higa Y, Morinaga O. J Nat Med. 2020 Sep; 74(4):710-721. Epub 2020 Jun 15.
- Review Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field.[J Food Sci. 2018]Review Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field.Hu Q, Zhou M, Wei S. J Food Sci. 2018 Jun; 83(6):1476-1483. Epub 2018 May 26.
- Review Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health.[Molecules. 2021]Review Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health.Haro-González JN, Castillo-Herrera GA, Martínez-Velázquez M, Espinosa-Andrews H. Molecules. 2021 Oct 22; 26(21). Epub 2021 Oct 22.
- Eugenol (Clove Oil) - LiverToxEugenol (Clove Oil) - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...