NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Eplerenone is an aldosterone receptor antagonist and potassium-sparing diuretic used in the therapy of hypertension. Eplerenone therapy has been associated with transient elevations in serum aminotransferase levels, but has yet to be linked to cases of clinically apparent drug induced liver disease.
Background
Eplerenone (e pler' e none) is a competitive antagonist of aldosterone at the mineralocorticoid receptor. The aldosterone receptor in the late distal tubules and collecting ducts of the kidneys induces sodium reabsorption and potassium excretion in the distal tubule. Inhibition of this receptor promotes a sodium diuresis, but maintains body potassium levels. Eplerenone has a higher affinity for the aldosterone receptor than spironolactone and is claimed to have fewer anti-androgenic effects (gynecomastia, hair loss). However, the two molecules are structurally quite similar. Eplerenone was approved for use in the United States in 2002 for treatment of hypertension and later for improving survival of stable patients with heart failure after myocardial infarction. Eplerenone is available in 25 and 50 mg tablets generically and under the brand name of Inspra. The typical dose of eplerenone is 25 or 50 mg once daily initially, with modification of the dose based upon blood pressure response and tolerance, maintenance doses ranging from 25 to 100 mg daily in one or two divided doses. Eplerenone is well tolerated and the most common side effects are hyperkalemia and increases in serum creatinine.
Hepatotoxicity
Eplerenone therapy has been associated with a low rate of serum aminotransferase elevations which are typically mild and transient. ALT elevations of greater than 3 times the ULN occurred in 0.7% and greater than 5 times in 0.2% of eplerenone treated compared to 0.3% and 0.3% of placebo treated subjects. Idiosyncratic, clinically apparent liver injury from eplerenone has yet to be reported. The similarity in structure to spironolactone suggests that it may share susceptibility to the acute liver injury reported rarely with that agent.
Likelihood score: E* (unproven but suspect rare cause of clinically apparent liver injury).
Mechanism of Injury
Eplerenone is metabolized in the liver by the cytochrome P450 system (CYP 3A4) and hepatic reactions may be generated by intermediates in its metabolism.
Outcome and Management
The mild serum aminotransferase elevations that have been reported with eplerenone resolved rapidly on discontinuation and in some instances resolved even with drug continuation. While yet unproven, cross reactivity to the liver injury that can occur with spironolactone should be assumed.
Drug Class: Diuretics, Potassium-Sparing Diuretics
Other Drugs in the Subclass: Amiloride, Spironolactone, Triamterene
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Eplerenone – Generic, Inspra®
DRUG CLASS
Diuretics
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Eplerenone | 107724-20-9 | C24-H30-O6 |
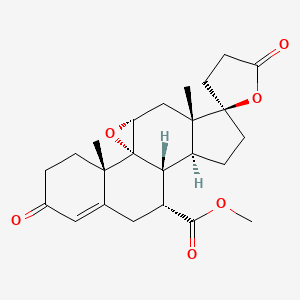
|
ANNOTATED BIBLIOGRAPHY
References updated: 13 October 2021
- Zimmerman HJ. Diuretic drugs. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 662-4.(Expert review of hepatotoxicity of diuretics published in 1999 mentions that clinically apparent liver injury due to diuretics is rare; hepatocellular jaundice has been reported with triamterene; no mention of eplerenone).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of cardiovascular agents, mentions that thiazide diuretics can rarely cause cholestatic hepatitis; no mention of potassium sparing diuretics).
- Jackson EK. Drugs affecting renal excretory function. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 445-70.(Textbook of pharmacology and therapeutics).
- Zillich AJ, Carter BL. Eplerenone—a novel selective aldosterone blocker. Ann Pharmacother. 2002;36:1567–76. [PubMed: 12243608](Review of pharmacology, mechanism of action, efficacy, and safety of eplerenone; no discussion of ALT elevations or hepatotoxicity).
- Burgess ED, Lacourcière Y, Ruilope-Urioste LM, Oparil S, Kleiman JH, Krause S, Roniker B, et al. Long-term safety and efficacy of the selective aldosterone blocker eplerenone in patients with essential hypertension. Clin Ther. 2003;25:2388–404. [PubMed: 14604739](Open label trial of eplerenone in 586 patients with hypertension; adverse events were mild including hypotension, impotence, nausea, dizziness, headache; ALT elevations in 5 patients [0.9%] led to withdrawal, but no details given and no mention of jaundice or symptoms).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 137 [0.5%] were done for idiosyncratic drug induced acute liver failure, none were attributed to a diuretic).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports of drug induced liver injury to a Spanish network found 570 cases; diuretics not mentioned as cause).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports [89% from the US]; no diuretic found in the 20 most commonly implicated agents).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury [24 with acute liver failure] due to drugs between 1993-1999 in Spain calculated relative risk of injury compared to the general population: hydrochlorothiazide was being taken by 7 and furosemide by 8 patients, but relative risk was not increased in comparison to a control group).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no case was attributed to a diuretic).
- Drugs for hypertension. Treat Guidel Med Lett. 2009;7:1–10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities: “thiazide diuretics are the first-line therapy for many patients with hypertension”).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen over a 12 year period at a large hospital in Bangalore, India, none were attributed to a diuretic).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, no diuretic was mentioned among the 30 most common causes of liver injury).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which none were attributed to a diuretic).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a diuretic).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to a diuretic).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to a diuretic).
- Drugs for hypertension. Med Lett Drugs Ther. 2020;62(1598):73–80. [PubMed: 32555118](Concise summary of efficacy, safety and costs of drug therapy of hypertension including the diuretics, focusing upon relative usefulness; no mention of hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy.[J Am Coll Cardiol. 2011]Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy.Rossignol P, Ménard J, Fay R, Gustafsson F, Pitt B, Zannad F. J Am Coll Cardiol. 2011 Nov 1; 58(19):1958-66.
- Review Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone.[Int J Cardiol. 2015]Review Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone.Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, Speziale G, Gaudio C. Int J Cardiol. 2015 Dec 1; 200:25-9. Epub 2015 May 21.
- The aldosterone antagonist and facultative diuretic eplerenone: a critical review.[Eur J Intern Med. 2005]The aldosterone antagonist and facultative diuretic eplerenone: a critical review.Reyes AJ, Leary WP, Crippa G, Maranhão MF, Hernández-Hernández R. Eur J Intern Med. 2005 Feb; 16(1):3-11.
- Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension.[J Am Soc Hypertens. 2008]Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension.Calhoun DA, White WB. J Am Soc Hypertens. 2008 Nov-Dec; 2(6):462-8. Epub 2008 Jul 23.
- Review The cardiovascular effects of eplerenone, a selective aldosterone-receptor antagonist.[Clin Ther. 2003]Review The cardiovascular effects of eplerenone, a selective aldosterone-receptor antagonist.Davis KL, Nappi JM. Clin Ther. 2003 Nov; 25(11):2647-68.
- Eplerenone - LiverToxEplerenone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...