NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The echinocandins include anidulafungin, caspofungin, and micafungin and are a relatively new class of antifungal agents that are administered parenterally and are used in therapy or prevention of serious, invasive aspergillosis and candidal infections. All three agents can cause transient and asymptomatic serum aminotransferase elevations, and individual instances of acute liver injury have been observed during therapy with these agents, but none has been definitely shown to cause clinically apparent acute drug induced liver injury.
Background
The echinocandins are a relatively new class of antifungal agents, whose activity is due to inhibition of glucan synthetase, the enzyme that is responsible for synthesis of β-1, 3-D-glucan, an essential component of the cell wall of filamentous fungi, such as Aspergillus and Candida species. This enzyme inhibition results in alteration in the fungal membrane integrity, followed by cell ballooning and, for Candida cells, lysis. Three echinocandins are available for use: caspofungin (kas" poe fun' jin), micafungin (mye" ka fun' jin) and anidulafungin (ay nid" ue la fun' jin). Caspofungin was approved for use in the United States in 2001, micafungin in 2005 and anidulafungin in 2006. All three are given intravenously and currently approved for therapy of esophageal candidiasis and for severe, disseminated or invasive candidiasis. Caspofungin is also approved for use as a secondary therapy of invasive aspergillosis and as empirical therapy for presumed fungal infection in febrile, neutropenic patients. Micafungin is also approved for prophylaxis against candidiasis in hematopoietic stem cell transplant recipients. Caspofungin is available under the brand name Cancidas in a formulation for intravenous use, the recommended dose in adults being 70 mg on the first day of treatment followed by 50 or 100 mg daily. Micafungin is available under the brand name of Mycamine in an intravenous formation, the recommended dosage being 50 mg daily for prophylaxis and 100 to 150 mg daily for therapy of candidemia. Anidulafungin is available under the brand name of Eraxis in intravenous formulations and the recommended dosage for disseminated candidal infection is 200 mg on day 1 followed by 100 mg daily. The echinocandins are administered intravenously in a slow infusion over one hour (to decrease the risk of acute infusion reactions). Common side effects include phlebitis and the histamine-like reaction marked by rash, urticaria, flushing, bronchospam, hypotension and facial swelling.
Hepatotoxicity
Transient elevations in liver enzymes have occurred in 2% to 15% of patients treated with the echinocandins, typically returning to baseline after withdrawal of therapy. Clinically apparent hepatotoxicity has occurred in isolated cases; however, a causal relationship to the antifungal agent is often difficult to prove, as these agents are typically used in persons who are critically ill and have other conditions that are associated with liver injury. The largest experience has been with caspofungin, which may have a higher rate of serum enzyme elevations than with micafungin or anidulafungin and has more frequently been linked to cases of acute, symptomatic liver injury. Nevertheless, the product label for all three echinocandins mentions adverse events of serum enzyme elevations, hepatitis and acute liver failure.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The cause of serum aminotransferase elevations during echinocandin therapy is unknown. In preclinical studies in animals, highest drug concentrations are found in the liver and a direct toxic effect or production of a toxic intermediate may be the cause of the abnormalities.
Outcome and Management
The liver injury seen with caspofungin, micafungin and anidulafungin rarely progresses beyond mild serum enzyme elevations. Caution should be taken in patients with preexisting hepatic insufficiency in whom the likelihood of severe acute liver injury appears to be greatest. Product labels for the echinocandins recommend monitoring of liver tests during therapy, particularly in patients with underlying liver disease. The effects of rechallenge and cross reactivity to hepatic injury among the echinocandins has not been evaluated.
Drug Class: Antifungal Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Anidulafungin – Eraxis®
Caspofungin – Cancidas®
Micafungin – Mycamine®
DRUG CLASS
Antifungal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Anidulafungin | 166663-25-8 | C58-H73-N7-O17 |
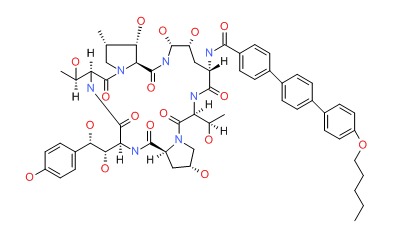 |
| Caspofungin | 162808-62-0 | C52-H88-N10-O15 |
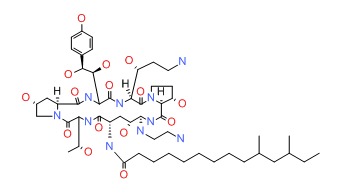 |
| Micafungin | 235114-32-6 | C56-H71-N9-O23-S |
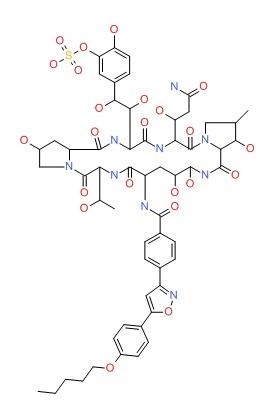 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 July 2017
- Zimmerman HJ. Antifungal agents. Hhepatic injury from antimicrobial agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 609-11.(Expert review of hepatotoxicity of antifungal agents published in 1999 before availability of the echinocandins).
- Moseley RH. Antifungal agents. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 470-3.(Review of hepatotoxicity of antifungal agents; caspofungin was the first of a new class of parenterally administered antifungal agents; mentions that elevations in ALT levels occur in 2-4% of patients during caspofungin therapy and that adverse reactions are less frequent with micafungin and anidulafungin).
- Bennett JE. Antifungal agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1571-93.(Textbook of pharmacology and therapeutics; the echinocandins have activity against fungal organisms acting to inhibit glucan synthesis; the 3 agents in clinical use have similar mechanism of action, but different pharmalogical properties).
- Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, et al.; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002; 347: 2020-9. [PubMed: 12490683](Controlled trial comparing caspofungin and amphotericin B in 224 adults with invasive candidiasis; similar efficacy but lower rate of side effects with caspofungin vs amphotericin: elevated ALT levels in 3.7% vs 8.1%, Alk P 8.3% vs 15.6%, bilirubin 2.8% vs 8.9%, but no serious hepatic adverse events attributed to either).
- Villanueva A, Gotuzzo E, Arathoon EG, Noriega LM, Kartsonis NA, Lupinacci RJ, Smietana JM, et al. A randomized double-blind study of caspofungin versus fluconazole for the treatment of esophageal candidiasis. Am J Med 2002; 113: 294-9. [PubMed: 12361815](Among 177 patients with esophageal candidiasis treated with either caspofungin or fluconazole for 14-42 days, serum ALT and Alk P elevations were more frequent with fluconazole [12% and 12%] than anidulafungin [5% and 9%], but no patient developed clinically apparent liver injury).
- Marr KA, Hachem R, Papanicolaou G, Somani J, Arduino JM, Lipka CJ, Ngai AL, et al. Retrospective study of the hepatic safety profile of patients concomitantly treated with caspofungin and cyclosporin A. Transpl Infect Dis 2004; 6: 110-6. [PubMed: 15569226](Retrospective study of 40 patients who received both caspofungin and cyclosporin A from 4 medical centers; 24 [60%] had at least one serum enzyme elevation and 4 [10%] stopped therapy because of hepatotoxicity; nevertheless, it was difficult to attribute the hepatic injury to caspofungin and “no severe hepatic adverse events were noted”).
- Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, Petersen FB, Sable CA, et al.; Caspofungin Salvage Aspergillosis Study Group. Efficacy and safety of Caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis 2004; 39: 1563-71. [PubMed: 15578352](Experience in treating 83 patients with invasive aspergillosis with caspofungin for an average of 28 days; ALT elevations occurred in only one patient and no serious drug related hepatic adverse events were reported).
- Wagner C, Graninger W, Presterl E, Joukhadar C. The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications. Pharmacology 2006; 78: 161-77. [PubMed: 17047411](Review of structure, pharmacology, clinical efficacy and safety of the echinocandins; elevations in ALT levels occur in up to 10% of patients treated with caspofungin, may be less with micafungin and anidulafungin).
- Bennett JE. Echinocandins for candidemia in adults without neutropenia. N Engl J Med 2006; 355: 1154-9. [PubMed: 16971721](Concise review on use of echinocandins in invasive fungal infections; ALT elevations occur in 11% of patients receiving caspofungin; isolated cases of clinically significant hepatic injury have occurred, “although it is not clear whether they were drug-related.”).
- Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, et al.; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007; 356: 2472-82. [PubMed: 17568028](Among 245 patients with invasive candidiasis treated with either caspofungin or fluconazole for 14-42 days, serum enzyme elevations were more frequent with fluconazole [7%] than anidulafungin [1.5%]).
- Cappelletty D, Eiselstein-McKitrick K. The echinocandins. Pharmacotherapy 2007; 27: 369-88. [PubMed: 17316149](Review of structure, pharmacokinetics, activity, clinical efficacy and side effects of the echinocandins; ALT elevations occur in 1-15% with caspofungin, 1-8% with micafungin, and 3-5% with anidulafungin).
- Anttila VJ, Salonen J, Ylipalosaari P, Koivula I, Riikonen P, Nikoskelainen J. A retrospective nationwide case study on the use of a new antifungal agent: patients treated with caspofungin during 2001-2004 in Finland. Clin Microbiol Infect 2007; 13: 606-12. [PubMed: 17378926](Retrospective analysis of 78 patients treated with caspofungin in Finland; 2 had severe hepatic toxicity, but the role of medication was uncertain as other factors were present, one patient died of acute liver failure after stopping caspofungin).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 8 were attributed to antifungal agents, including 4 to terbinafine, 2 to fluconazole, 1 each to ketaconazole and itraconazole but none to the echinocandins).
- Fortún J, Martín-Dávila P, Montejo M, Muñoz P, Cisneros JM, Ramos A, Aragón C, et al.; GESITRA Study Group. Prophylaxis with caspofungin for invasive fungal infections in high-risk liver transplant recipients. Transplantation 2009; 87: 424-35. [PubMed: 19202450](Experience in using caspofungin for 21-90 days as prophylaxis against invasive fungal infections in 71 high risk liver transplant recipients; ALT elevations were frequent, but difficult to relate to drug because of the underlying condition).
- Zaoutis TE, Jafri HS, Huang LM, Locatelli F, Barzilai A, Ebell W, Steinbach WJ, et al. A prospective, multicenter study of caspofungin for the treatment of documented Candida or Aspergillus infections in pediatric patients. Pediatrics 2009; 123: 877-84. [PubMed: 19255017](Open label study of caspofungin for 2 to 87 days in 49 children with invasive fungal infections; ALT elevations occurred in 14.6%, all resolved despite continuation of therapy and none were clinically apparent).
- Antifungal drugs. Treat Guidel Med Lett 2009; 7: 95-102. [PubMed: 19940816](Concise summary of therapy of fungal infections with recommendations on agents, dosage and duration of treatment and safety; mentions that the echinocandins all have mild hepatic toxicities).
- Menichetti F. Anidulafungin, a new echinocandin: effectiveness and tolerability. Drugs 2009; 69 Suppl 1: 95-7. [PubMed: 19877741](Review of efficacy and safety of anidulafungin mentions that adverse events are uncommon, but include increases in GGT, ALT and AST levels).
- King KY, Edwards MS, Word BM. Hepatitis associated with micafungin use in a preterm infant. J Perinatol 2009; 29: 320-2. [PubMed: 19325554](Female premature newborn [HCV- and HIV-exposed] developed elevation in liver tests starting on day 2 of micafungin therapy, with peak on day 16 [bilirubin 16.3 mg/dL, ALT 860 U/L], liver tests improved gradually following discontinuation of drug).
- Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother 2010; 54: 2409-19. [PMC free article: PMC2876415] [PubMed: 20308378](Systematic review of 39 controlled trials in more than 8000 patients, found liver enzyme elevations in 14.5% of patients on amphotericin (pooled estimate); 19.7% on voriconazole; 18.9% itraconazole; 10% fluconazole; 2.8% anidulafungin; 7.2% caspofungin; and 5.7% micafungin).
- Arrieta AC, Maddison P, Groll AH. Safety of micafungin in pediatric clinical trials. Pediatr Infect Dis J 2011; 30: e97-e102. [PubMed: 21378595](Review of 6 clinical trials of micafungin in pediatric patients found 3% of 296 children developed “treatment related” ALT elevations, but no mention was made of clinically apparent liver injury).
- Cornely OA, Pappas PG, Young JA, Maddison P, Ullmann AJ. Accumulated safety data of micafungin in therapy and prophylaxis in fungal diseases. Expert Opin Drug Saf 2011; 10: 171-83. [PubMed: 21306282](Review of pooled adverse event data from 17 clinical studies of micafungin in 3028 patients found 8.6% had treatment related hepatic adverse events, 2% had ALT elevations, 1% hyperbilirubinemia, 0.5% required treatment discontinuation, and 0.1% had hepatic failure, but no specifics given).
- Valerio M, Muñoz P, Bouza E. [Liver toxicity of micafungin. Is this drug safe?]. Enferm Infecc Microbiol Clin 2011; 29 Suppl 2: 29-32. Spanish. [PubMed: 21420574](Review of safety of micafungin, mentions that ALT elevations occur in 7% of patients treated with caspofungin, 3% with micafungin and 2% with anidulafungin).
- Doria C, Bodzin AS, Vaccino S, Daskalakis C, Krawitz S, Ramirez CB. A retrospective analysis of the use of caspofungin in recipients of liver transplant with a modified high index of suspicion for fungal infection. A critical review of mortality, acute cellular rejection, infections, and changes in the liver function tests while on caspofungin. Clin Transplant 2011; 25: 569-75. [PubMed: 20662881](Among 82 liver transplant recipients, 16 received caspofungin in the early posttransplant period for suspected invasive fungal infection, 3 [13%] of whom had transient rise in ALT or AST, but none of whom required dose modification).
- Yamaguchi M, Kurokawa T, Ishiyama K, Aoki G, Ueda M, Matano S, Takami A, et al. Efficacy and safety of micafungin as an empirical therapy for invasive fungal infections in patients with hematologic disorders: a multicenter, prospective study. Ann Hematol 2011; 90: 1209-17. [PubMed: 21695388](Among 121 adults with hematologic disorders treated with micafungin for either febrile neutropenia or suspected invasive fungal infection, 4 [3.3%] developed ALT elevations and one developed jaunidice).
- Goto N, Hara T, Tsurumi H, Ogawa K, Kitagawa J, Kanemura N, Kasahara S, et al. Efficacy and safety of micafungin for treating febrile neutropenia in hematological malignancies. Am J Hematol 2010; 85: 872-6. [PubMed: 20882524](Among 53 patients with febrile neutropenia treated with micafungin, 6 developed AST elevations, but all were transient, mild [<3 times ULN], and asymptomatic).
- Wang LL, Zhuge X, Xiao GH, Zhang Y. Hepatic safety of caspofungin during treatment of invasive fungal diseases in elderly patients. Chin Med J (Engl) 2012; 125: 2240. [PubMed: 22884162](Among 50 patients above 80 years of age treated with caspofungin for invasive fungal disease, 21 had abnormal liver tests before treatment, of whom 3 [14%] worsened on therapy [1 mild and 2 severe] compared to 2 of 29 with normal liver tests initially, of whom 2 worsened [7%], but both were mild).
- León-Gil C, Ubeda-Iglesias A, Loza-Vázquez A, de la Torre MV, Raurich-Puigdevall JM, Alvarez-Sánchez B, Ortiz-Leyva C, et al; ProCAS Study Group. Efficacy and safety of caspofungin in critically ill patients. ProCAS Study. Rev Esp Quimioter 2012; 25: 274-82. [PubMed: 23303260](Among 98 critically ill patients with severe fungal infections treated with caspofungin for 9-21 days, there were no significant changes in serum bilirubin, ALT or Alk P levels and no liver related serious adverse events).
- Ruhnke M, Paiva JA, Meersseman W, Pachl J, Grigoras I, Sganga G, Menichetti F, et al. Anidulafungin for the treatment of candidaemia/invasive candidiasis in selected critically ill patients. Clin Microbiol Infect 2012; 18: 680-7. [PMC free article: PMC3510306] [PubMed: 22404732](Among 216 critically ill patients with invasive fungal infections treated with anidulafungin for 10 to 42 days, 3 [1.7%] developed AST elevations above 5 times ULN, but there were no liver related serious adverse events).
- Kohno S, Izumikawa K, Yoshida M, Takesue Y, Oka S, Kamei K, Miyazaki Y, et al. A double-blind comparative study of the safetyand efficacy of caspofungin versus micafungin in the treatment of candidiasis andaspergillosis. Eur J Clin Microbiol Infect Dis 2013; 32: 387-97. [PMC free article: PMC3569581] [PubMed: 23052987](Among 121 patients with invasive fungal infections randomized to receive either micafungin or caspofungin, ALT elevations occurred in 8.3% of caspofungin vs 6.7% of micafungin treated patients and were above 5 times ULN in 0% vs 3%, but no patient developed clinically apparent liver injury with jaundice).
- Kobayashi R, Suzuki N, Yoshida M, Iizuka S, Suzuki D, Sano H, Kudoh T. Efficacy and safety of micafungin for febrile neutropenia in pediatric patients with hematological malignancies: a multicenter prospective study. J Pediatr Hematol Oncol 2013; 35: e276-9. [PubMed: 23743960](Among 25 children with 30 episodes of febrile neutopenia after cancer chemotherapy who were treated with micafungin for 6 to 47 days, ALT elevations occurred in 2, but were transient and mild, not requiring dose modification).
- Yamazaki S, Nakamura F, Yoshimi A, Ichikawa M, Nannya Y, Kurokawa M. Safety of high-dose micafungin for patients with hematological diseases. Leuk Lymphoma 2014 Mar 7. [Epub ahead of print] [PubMed: 24460099](Retrospective analysis of safety of two doses of micafungin for invasive fungal infections, found any elevation in ALT in 26% of 58 recipients of standard dose [150 mg/day: n=58] vs 27% of 26 recipients of high dose [300 mg/day], and elevations above 5 times ULN in only 1 patient in each group).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to an echinocandin).
- Jung DS, Tverdek FP, Jiang Y, Kontoyiannis DP. Switching to anidulafungin from caspofungin in cancer patients in the setting of liver dysfunction is associated with improvement of liver function tests. J Antimicrob Chemother 2015; 70: 3100-6. [PubMed: 26311837](In a retrospective study of 61 patients who developed abnormal liver tests during treatment with caspofungin and were then switched to anidulafungin, values improved overall, mean ALT falling from 132 to 71 U/L and bilirubin from 2.5 to 2.2 mg/dL, suggesting that anidulafungin is less hepatotoxic than caspofungin).
- Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 2017; 16: 149-65. [PubMed: 27927037](Review of the hepatotoxicity of antifungal agents discusses the frequency of serum enzyme elevations during anidulafungin, caspofungin and micafungin therapy but not clinically apparent liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Echinocandin antifungal drugs in fungal infections: a comparison.[Drugs. 2011]Review Echinocandin antifungal drugs in fungal infections: a comparison.Chen SC, Slavin MA, Sorrell TC. Drugs. 2011 Jan 1; 71(1):11-41.
- Review Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin and its impact on use and costs: review of the literature.[Eur J Med Res. 2011]Review Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin and its impact on use and costs: review of the literature.Wilke M. Eur J Med Res. 2011 Apr 28; 16(4):180-6.
- Review Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature.[Eur J Med Res. 2011]Review Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis: review of the literature.Kofla G, Ruhnke M. Eur J Med Res. 2011 Apr 28; 16(4):159-66.
- Risk factors of mortality and comparative in-vitro efficacy of anidulafungin, caspofungin, and micafungin for candidemia.[J Microbiol Immunol Infect. 2014]Risk factors of mortality and comparative in-vitro efficacy of anidulafungin, caspofungin, and micafungin for candidemia.Lee SC, Lee CW, Shih HJ, Huang SH, Chiou MJ, See LC. J Microbiol Immunol Infect. 2014 Jun; 47(3):245-53. Epub 2013 Nov 15.
- In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance.[J Clin Microbiol. 2008]In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. J Clin Microbiol. 2008 Jan; 46(1):150-6. Epub 2007 Nov 21.
- Echinocandins - LiverToxEchinocandins - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...