NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Doxycycline is a semisynthetic, tetracycline related bacteriostatic antibiotic that has been linked to rare instances of acute cholestatic liver injury.
Background
Doxycycline (dox" i sye' kleen) is a semisynthetic tetracycline that is used for mild-to-moderate infections due to susceptible organisms. Doxycycline, like other tetracyclines, is active against a wide spectrum of gram-positive and gram-negative bacteria as well as against several rickettsia, spirochetes, chlamydia and mycoplasma. Unlike tetracycline and oxytetracycline, doxycycline has excellent oral availability and wide tissue penetration. Indications include upper respiratory, skin, or soft tissue infections due to susceptible bacteria, gonorrhea and syphilis in penicillin-allergic patients, non-gonococcal urethritis, acute pelvic inflammatory disease, epididymitis, oorchitis, Lyme disease, and as prophylaxis against traveler’s diarrhea. Doxycycline is also used chronically as treatment of acne. Doxycycline was approved for use in the United States in 1967 and is still widely used, with more than 11 million prescriptions filled yearly. Doxycycline is available in multiple generic forms in capsules and tablets ranging from 20 to 100 mg, and as oral suspensions for pediatric use. Typical adult doses are 100 to 200 mg twice daily for 7 to 30 days. Parenteral forms for intravenous or intramuscular administration are also available. Trade names for doxycycline include Vibramycin, Oracea, Adoxa, Monodox and Doxycin. Common side effects include headache, dizziness, nausea, gastrointestinal upset, skin and tooth discoloration and rash.
Hepatotoxicity
Doxycycline has been associated with rare instances of hepatic injury, generally arising within 1 to 2 weeks of starting therapy, sometimes with a history of previous administration of the agent without injury. The pattern of injury ranges from hepatocellular to cholestatic and is probably most commonly mixed. The onset is often abrupt and can be accompanied by signs of hypersensitivity, such as fever, rash and eosinophilia (DRESS syndrome). Recovery is usually rapid and usually complete within 4 to 6 weeks. However, instances of severe and prolonged cholestatic liver injury have been reported with oral doxycycline. The autoimmune-like hepatitis that has been described with minocycline has not been linked to doxycycline, despite similarities in chemical structure and similar indications and uses, perhaps because it is used less frequency in a low dose, long term regimen. High dose intravenous doxycycline can cause acute fatty liver typical of that caused by intravenous tetracycline, particularly in susceptible patients such as pregnant women. This type of injury is, however, quite rare. Nevertheless, for these reasons, the duration and dose of parenteral doxycycline therapy should be minimized.
Likelihood score: B (highly likely but rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the idiosyncratic liver injury associated with doxycycline is unknown, but several features (short latency, recurrence with reexposure) suggest an immunoallergic etiology. The injury is similar to what has been described much more frequency with minocycline which has been linked to HLA-B*35:01, although only in a minority of cases.
Outcome and Management
Recovery after withdrawal of doxycycline is usually rapid, but in instances with severe cholestasis, can require 2 to 6 months. No case of acute liver failure has been reported due to doxycycline, but rare instances of vanishing bile duct syndrome have been linked to its use. Cross sensitivity to hepatic injury between minocycline and doxycycline has not been shown, but both can cause a short incubation period acute hepatitis with immunoallergic features, so that some degree of cross reactivity may occur.
Drug Class: Antiinfective Agents, Tetracyclines
CASE REPORT
Case 1. Cholestatic hepatitis attributed to doxycycline therapy.
[Modified from: Björnsson E, Lindberg J, Olsson R. Liver reactions to oral low-dose tetracyclines. Scand J Gastroenterol 1997; 32: 390-5.]
A 32 year old man with fever and cough was treated with doxycycline for suspected pneumonitis and developed skin rash, abdominal pain and dark urine one day later. Symptoms of fatigue, nausea and pruritus arose and doxycycline was stopped after 8 days of therapy. His serum enzymes were elevated and he was mildly jaundiced with a total bilirubin of 4.4 mg/dL (Table). He also had eosinophilia (13.5%). He tested negative for antibodies to hepatitis A, B, and C as well as markers of acute CMV and Epstein Barr infection. Autoantibodies were negative, and an abdominal ultrasound was normal. He remained jaundiced for several months and underwent a liver biopsy that showed intrahepatic cholestasis with mild inflammatory changes suggestive of drug induced liver injury. In follow-up at 3 and again at 18 months, all liver tests had returned to normal.
Key Points
| Medication: | Doxycycline (200 mg once daily for 8 days) |
|---|---|
| Pattern: | Mixed (R=3.9), later hepatocellular (R=10) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 1 day to symptoms, 8 days to jaundice |
| Recovery: | 3 months |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Doxycycline given for 8 days for suspected pneumonia | |||||
| 1 day | 0 | NA | NA | NA | Rash and nausea |
| 8 days | 0 | 173 | 130 | 4.4 | Doxycycline stopped |
| 11 days | 3 days | 194 | 112 | 5.0 | Negative viral markers |
| 36 days | 28 days | 431 | 104 | 8.1 | |
| 53 days | 45 days | 442 | 91 | 7.1 | Liver biopsy |
| 56 days | 48 days | 474 | 104 | 7.2 | |
| 2 months | 2 months | 743 | 100 | 4.6 | |
| 3 months | 3 months | 388 | 87 | 3.1 | |
| 4 months | 4 months | 32 | 61 | 0.8 | |
| Normal Values | <40 | <115 | <1.2 | ||
* Estimated from Figure 1 and converted from µkat/l and µmol/L.
Comment
A convincing history, presentation, biochemical pattern and course for drug induced liver disease with a rapid onset, mixed followed by hepatocellular pattern of enzyme elevations and fairly prolonged jaundice. The only medication taken was doxycycline, which the patient had received at least twice in the past. The short latency to onset suggests previous sensitization. Recovery was slow but complete. Thus, the overall presentation is quite convincing as immunoallergic drug induced liver injury due to doxycycline. Future exposures to doxycycline as well as other tetracyclines should be avoided.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Doxycycline – Generic, Monodox®, Vibramycin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
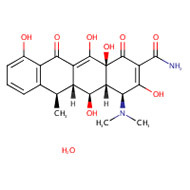
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Doxycycline Monohydrate | 17086-28-1 | C22-H24-N2-O8.H2-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 23 January 2019
- Zimmerman HJ. Tetracyclines. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999. p. 599-602.(Expert review of tetracycline and liver injury published in 1999; the tetracyclines cause two forms of drug induced liver injury, microvesicular fat and liver failure occurring after 4-10 days with high does of parenteral tetracyclines and an idiosyncratic liver injury that occurs with the oral agents, doxycycline causing a cholestatic and minocycline a hepatocellular injury which may be associated with autoimmune features).
- Moseley RH. Tetracyclines. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 468.(Expert review of tetracycline induced liver injury mentions that the hepatotoxicity of intravenous tetracycline is of historic interest only as it is no longer given parenterally; both doxycycline and minocycline have been associated with idiosyncratic liver injury).
- MacDougall C, Chambers HF. Tetracyclines and glycylcyclines. Protein synthesis inhibitors and miscellaneous antibacterial agents. In, Brunton LL, Chabner KA, Knollman KC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1521-6.(Textbook of pharmacology and therapeutics).
- Wruble LD, Ladman AJ, Britt LG, Cummins AJ. Hepatotoxicity produced by tetracycline overdosage. JAMA 1965; 192: 6-8. [PubMed: 14262279](56 year old woman with cecal volulus developed jaundice [bilirubin 7.8 mg/dL, AST 98 U/L, Alk P normal] 2 days after receiving 8 g of tetracycline, subsequently progressing to liver failure and death; autopsy showed fatty liver).
- Tetracyclines and the liver in pregnancy. Lancet 1966; 1: 357-8. [PubMed: 4159865](Editorial on the history of acute fatty liver of pregnancy, first described by Sheehan in 1940 and later linked to high dose iv tetracycline in pregnancy, but also in nonpregnant women and in men).
- Wenk RE, Gebhardt FC, Bhagavan BS, Lustgarten JA, McCarthy EF. Tetracycline-associated fatty liver of pregnancy, including possible pregnancy risk after chronic dermatologic use of tetracycline. J Reprod Med 1981; 26: 135-41. [PubMed: 7230149](Two cases of acute fatty liver over a 15 year period and 60,000 deliveries; one woman received tetracycline for 3 days, the second had no history of its use, but had tetracycline detected in bony tissue suggestive of chronic use).
- Böcker R, Estler CJ, Müller S, Pfandzelter C, Spachmüller B. Comparative evaluation of the effects of tetracycline, rolitetracycline and doxycycline on some blood parameters related to liver function. Arzneimittelforschung 1982; 32: 237-41. [PubMed: 7200783](Minocycline caused a dose dependent rise in AST and increase in triglycerides, but no hepatic histological changes in mice; similar findings were previously reported with tetracycline).
- Schrumpf E, Nordgard K. Unusual cholestatic hepatotoxicity of doxycycline in a young male? Scand J Gastroenterol 1986; 21 (Suppl 120): 68. Not in PubMed.(Abstract: Patient developed severe and prolonged cholestatic hepatitis after 2 weeks of oral doxycycline [bilirubin 9.8 rising to 69.5 mg/L, ALT 53 to 132 U/L, Alk P 283 to 775 U/L] and severe itching; ultimate recovery after 6 months).
- Lienart F, Morissens M, Jacobs P, Ducobu J. Doxycycline and hepatotoxicity. Acta Clin Belg 1992; 47: 205-8. [PubMed: 1332350](42 year old woman with severe cardiomyopathy developed encephalopathy and enzyme elevations with jaundice after 5 days of oral doxycycline [bilirubin 1.2 mg/dL, ALT 139 U/L, Alk P 373 U/L, ammonia 99 mcg/dL], resolving rapidly on stopping; likely due to heart failure and ischemia rather than drug induced liver injury).
- Carson JL, Strom BL, Duff A, et al. Acute liver disease associated with erythromycins, sulfonamides, and tetracyclines. Ann Intern Med 1993; 119 (7 Pt 1): 576-83. [PubMed: 8363168](Case control study using Medicaid billing results between 1980-87 found 107 cases of hospitalization for unexplained hepatitis, odds ratios for erythromycin 5.2; sulfonamides 11.4; tetracyclines 5.2; total of 5 cases exposed to tetracycline, doxycycline or minocycline).
- Hunt CM, Washington K. Tetracycline-induced bile duct paucity and prolonged cholestasis. Gastroenterology 1994; 107: 1844-7. [PubMed: 7958700](Two cases of severe and prolonged cholestatic hepatitis and bile duct paucity after oral tetracyclines; 37 year old woman developed jaundice 2 days after 3 day course of doxycycline with prolonged cholestasis [peak bilirubin 30 mg/dL], but ultimate recovery; 63 year old woman developed jaundice 6 weeks after a 2 week course of tetracycline [bilirubin 11.8 mg/dL, ALT 245 U/L], with prolonged jaundice [peak bilirubin 29.5 mg/dL) and persistence of enzyme elevations for >3 years [Alk P 631 U/L, ALT 97 U/L]).
- Friis H, Andreasen PB. Drug-induced hepatic injury: an analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J Intern Med 1992; 232: 133-8. [PubMed: 1506809](Adverse drug reaction reports between 1978 and 1987 in Denmark; no tetracycline is mentioned as a cause).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years’ experience. N Z Med J 1996; 109: 315-9. [PubMed: 8816722](Adverse drug reaction reports identified 943 liver injuries over 21 years in New Zealand; triacetyloleandomycin accounted for 21 cases [2.1%] and minocycline for at least 4).
- Tham SN, Kwok YK, Chan HL. Cross-reactivity in fixed drug eruptions to tetracyclines. Arch Dermatol 1996; 132: 1134-5. [PubMed: 8795565](Among 9 Chinese patients with a fixed drug eruption due to tetracycline, all had recurrence on doxycycline and 3 on minocycline; no mention of liver involvement).
- Björnsson E, Lindberg J, Olsson R. Liver reactions to oral low-dose tetracyclines. Scand J Gastroenterol 1997; 32: 390-5. Review. [PubMed: 9140164](32 year old man developed abdominal pain, dark urine and rash within 24 hours of starting doxycycline which he had received in the past [bilirubin 4.3- 8.1 mg/dL, ALT 3.5 times ULN, Alk P 1.1 times ULN], resolving within 3 months of stopping; thorough review of all published and SADRAC reported cases of oral tetracycline associated liver injury found 15 cases, only 6 rated as likely, none with tetracycline, 5 doxycycline, 1 lymecycline).
- Shapiro LE, Knowles SR, Shear NH. Comparative safety of tetracycline, minocycline, and doxycycline. Arch Dermatol 1997; 133: 1224-30. [PubMed: 9382560](Review of toxicity of tetracyclines from literature and a Canadian database; minocycline had the highest rates of adverse events, but all were relatively safe: no lupus-like syndrome associated with doxycycline or tetracycline).
- Westermann GW, Böhm M, Bonsmann G, Rahn KH, Kisters K. Chronic intoxication by doxycycline use for more than 12 years. J Intern Med 1999; 246: 591-2. [PubMed: 10620103](38 year old man took large doses of doxycycline chronically and developed repeated bouts of mild jaundice [bilirubin 3.4-5.0 mg/dL, ALT 44-118 U/L, Alk P 466 U/L] and weakness, skin discoloration and heart block, resolving upon stopping).
- Sturkenboom MC, Meier CR, Jick H, Stricker BH. Minocycline and lupuslike syndrome in acne patients. Arch Intern Med 1999; 159: 493-7. [PubMed: 10074958](Case control study in 27,688 acne patients in UK database, risk of lupus increased 8.5 fold in minocycline treated patients, not with other tetracyclines, typically long term therapy; liver involvement not mentioned; more common in women, absolute risk low).
- Firouzmand M, Zafrani ES, Dhumeaux D, Mallat A. [Microvesicular steatosis after administration of therapeutic doses of doxycycline] Gastroenterol Clin Biol 2002; 26: 1176-7. French. [PubMed: 12520208](65 year old woman with alcoholic liver disease developed jaundice and mild increases in ALT and Alk P when given 1 month course of doxycycline; liver biopsy showed fat, much was microvesicular, but patient was also on ofloxacillin, rifampin and recently received ampicillin and gentamicin for osteomyelitis).
- Selimoglu MA, Ertekin V. Autoimmune hepatitis triggered by Brucella infection or doxycycline or both. Int J Clin Pract 2003; 57: 639-41. [PubMed: 14529072](11 year old girl was found to have abnormal liver tests 3 weeks after finishing a 6 week course of doxycycline for acute brucellosis [bilirubin 1.2 mg/dL, ALT 222 U/L, Alk P 561 U/L, ANA negative, HLA B8 DRB1*03], a liver biopsy showing inflammation, necrosis and bridging fibrosis, improving on prednisone therapy, relapsing on stopping and ultimately requiring long term azathioprine therapy).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, none of which were attributed to minocycline, doxycycline or tetracycline).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-1101. [PubMed: 16165719](Among 103 cases of fulminant drug induced liver injury reported to a Swedish registry between 1966 and 2002, one case was attributed to doxycycline, but no other tetracycline mentioned).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, Garcia-Munoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology 2005; 129: 512-21. [PubMed: 16083708](Reports to a Spanish network found 461 cases of drug induced liver disease; no tetracycline was listed among the top 20 agents implicated [at least 5 cases]).
- Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther 2005; 27: 1329-42. [PubMed: 16291409](Systematic review of overall safety of doxycycline and minocycline stressing overall low rate of adverse events [13-72 per million prescriptions]; little mention of liver toxicity).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33-8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports – 89% from the US; the list of most 21 most commonly implicated drugs did not include a tetracycline).
- Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. Br J Dermatol 2007; 157: 540-6. [PubMed: 17596147](Analysis of UK database on 97,694 subjects with acne, 25% received minocycline, 16% doxycycline and 45% other tetracyclines. Hazard ratio for lupus-like syndrome was 3.11 for minocycline; no increased risk or association with doxycycline or other tetracyclines).
- Heaton PC, Fenwick SR, Brewer DE. Association between tetracycline or doxycycline and hepatotoxicity: a population based case-control study. J Clin Pharm Ther 2007; 32: 483-7. [PubMed: 17875115](Analysis of 2 years of Medicaid claims in California found 3377 cases of “hepatotoxicity”; 20 had received tetracycline <45 days before onset; only 4 controls had: adjusted odds ratio 3.7; not elevated for doxycycline; this despite safety record of oral tetracyclines and known hepatotoxicity of doxycycline).
- Hempel U, Schäffler A, Salzberger B, Rümmele P, Schölmerich J. [A 42-year-old farmer with nonspecific leukocytosis and elevated transaminases. Acute septic reaction in Coxiella burnetii infection.] Internist (Berl) 2008; 49: 743-6. German. [PubMed: 18309471]
- Meropol SB, Chan KA, Chen Z, Finkelstein JA, Hennessy S, Lautenbach E, Platt R, et al. Adverse events associated with prolonged antibiotic use. Pharmacoepidemiol Drug Saf 2008 17: 423-32. [PMC free article: PMC4269235] [PubMed: 18215001](Analysis of 3 large UK and US databases on medication use and clinical outcomes covering ~6 million patients for adverse events related to >1 month of either amoxicillin [0-1.2/100,000], ciprofloxacin [0-5.7/100,000] and doxycycline [0-0.9/100,000 person days], but no mention of liver toxicities).
- Carrascosa MF, Lucena MI, Andrade RJ, Caviedes JRS, et al. Fatal acute hepatitis after sequential treatment with levofloxacin, doxycycline, and naproxen in a patient presenting with acute Mycoplasma pneumoniae infection. Clin Ther 2009; 31: 1014-19. [PubMed: 19539102](63 year old man developed acute liver injury 1 week after starting a 3 day course of levofloxacin and 5 days after starting doxycycline [bilirubin 4.0 rising to >35 mg/dL, ALT 1577 U/L, Alk P 189 U/L], with subsequent progressive liver failure and death; biopsy showed massive necrosis).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, minocycline accounted for 3 cases, doxycycline for 3 cases and tetracycline was listed as a secondary possible cause for one).
- Chavant F, Lafay-Chebassier C, Beauchant M, Perault-Pochat MC. [Doxycycline induced hepatitis]. Gastroenterol Clin Biol 2008; 32: 825-7. French. [PubMed: 18823729](37 year old man developed fatigue 6 weeks after starting doxycycline [bilirubin 2.8 mg/dL, ALT 2322 U/L, Alk P 184 U/L, ANA 1:100], resolving within 6 weeks of stopping).
- Robles DT, Leonard JL, Compton N, Waghmare A, McDonough KA, George E, Wolgamot G, Fleckman P. Severe drug hypersensitivity reaction in a young woman treated with doxycycline. Dermatology 2008; 217: 23-6. [PubMed: 18332631](20 year old woman developed severe rash and fever 3 weeks after starting doxycycline for acne [bilirubin 2.5 mg/dL, ALT 133 U/L, Alk P 205 U/L, eosinophils 980/µL], requiring intubation and corticosteroid therapy and 3 week hospitalization, ultimately resolving).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database containing 9036 hepatic adverse drug reactions in children includes 117 cases attributed to minocycline, but no other tetracycline listed in the top 40 causes).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol 2010; 105: 2396-404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; none were due to tetracyclines).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 25 to antituberculosis agents, including 15 to isoniazid alone [ranking first], 6 to isoniazid combined with other agents, 3 to rifampin and pyrazinamide, and 1 to dapsone).
- Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 2010; 51: 2040-8. [PubMed: 20512992](Among 261 cases of autoimmune hepatitis seen at the Mayo Clinic between 1997 and 2007, 24 were attributed to drugs, 11 to minocylcine, 11 to nitrofurantoin and 2 to others; all responded to corticosteroid therapy and all who were withdrawn did not relapse).
- Mailhol C, Tremeau-Martinage C, Paul C, Godel A, Lamant L, Giordano-Labadie F. [Severe drug hypersensitivity reaction (DRESS syndrome) to doxycycline]. Ann Dermatol Venereol 2010; 137: 40-3. French. [PubMed: 20110067](59 year old woman developed fever, rash and facial edema 3 weeks after starting doxycycline for malaria prophylaxis [bilirubin normal, ALT 278 U/L, eosinophils 15%, atypical lymphocytosis], resolving on discontinuation).
- Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical. Infection 2010; 38: 3-11. [PubMed: 20107858](Review of hepatotoxicity of antibiotics; mentions that hepatotoxicity from oral tetracycline is rare ~1.5 cases per million prescriptions, whereas minocycline has been associated with either an immediate reaction with eosinophilia, dermatitis and enzyme elevations or a delayed autoimmune hepatitis-like syndrome).
- Glenn C, Feldman SR. Letter: Tetracycline-induced hepatotoxicity. Dermatol Online J 2011; 17: 14. [PubMed: 22233750](49 year old woman developed fatigue, edema, low albumin and raised alkaline phosphatase levels [bilirubin and ALT normal] 6 months after starting oral tetracycline for rosacea, albumin and alkaline phosphatase levels returning to normal upon stopping, later tolerating doxycycline).
- Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci 2011; 56: 958-76. (Review of drug induced autoimmune hepatitis-like syndromes, most commonly caused by nitrofurantoin and minocycline, but also with hydralazine, methyldopa and more rarely with statins, fibrates, NSAIDs, various herbals and tumor necrosis factor antagonists). [PubMed: 21327704]
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to doxycycline or other tetracyclines).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 37 of which were attributed to an antibiotic, but none to a tetracycline).
- Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Sarganas G, Kreutz R, et al. Drug-induced liver injury: results from the hospital-based Berlin Case-Control Surveillance Study. Br J Clin Pharmacol 2015; 79: 988-99. [PMC free article: PMC4456131] [PubMed: 25444550](Among 76 inpatients with hepatitis of uniknown cause enrolled in a prospective case-cohort surveillance study between 2002 and 2011, one was attributed to doxycycline, but no other tetracycline was implicated).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics including 28 [3%] to minocycline and 4 [0.4%] to doxycycline).
- Tang DM, Koh C, Twaddell WS, von Rosenvinge EC, Han H. Acute hepatocellular drug-induced liver injury from bupropion and doxycycline. ACG Case Rep J 2015; 3: 66-8. [PMC free article: PMC4612764] [PubMed: 26504884](29 year old African American man developed jaundice 14 days after starting bupropion and doxycycline [bilirubin 19.9 mg/dL, ALT 888 U/L, Alk P 234 U/L, INR 3.0, eosinophils 5%] with resolution within 2 months on prednisone, the authors suggesting that bupropion was the most likely cause).
- Urban TJ, Nicoletti P, Chalasani N, Serrano J, Stolz A, Daly AK, Aithal GP, et al; Drug-Induced Liver Injury Network (DILIN); Pharmacogenetics of Drug-Induced Liver Injury group (DILIGEN); International Serious Adverse Events Consortium (iSAEC). Minocycline hepatotoxicity: clinical characterization and identification of HLA-B∗35:02 as a risk factor. J Hepatol 2017; 67: 137-44. [PMC free article: PMC5634615] [PubMed: 28323125](The uncommon HLA allele B*35:02 was found in 4 of 25 [16%] of patients with minocycline hepatotoxicity vs 0.6% in a population control group; those with and without this allele did not different in clinical features or outcome).
- Doxycycline-induced cholestatic liver injury.[Clin J Gastroenterol. 2021]Doxycycline-induced cholestatic liver injury.Varma S, Nathanson J, Dowlatshahi M, Del Portillo A, Ramirez I, Garcia-Carrasquillo R. Clin J Gastroenterol. 2021 Oct; 14(5):1503-1510. Epub 2021 Jul 6.
- A pharmacovigilance study of the association between tetracyclines and hepatotoxicity based on Food and Drug Administration adverse event reporting system data.[Int J Clin Pharm. 2022]A pharmacovigilance study of the association between tetracyclines and hepatotoxicity based on Food and Drug Administration adverse event reporting system data.Wei C, Liu Y, Jiang A, Wu B. Int J Clin Pharm. 2022 Jun; 44(3):709-716. Epub 2022 Apr 1.
- Review Demeclocycline.[LiverTox: Clinical and Researc...]Review Demeclocycline.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Doxycycline and minocycline in Helicobacter pylori treatment: A systematic review and meta-analysis.[Helicobacter. 2021]Review Doxycycline and minocycline in Helicobacter pylori treatment: A systematic review and meta-analysis.Zhao J, Zou Y, Li K, Huang X, Niu C, Wang Z, Zhao S, Zhang Y, Song C, Xie Y. Helicobacter. 2021 Oct; 26(5):e12839. Epub 2021 Jul 28.
- Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi.[Ticks Tick Borne Dis. 2010]Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi.Ates L, Hanssen-Hübner C, Norris DE, Richter D, Kraiczy P, Hunfeld KP. Ticks Tick Borne Dis. 2010 Mar; 1(1):30-4. Epub 2010 Jan 7.
- Doxycycline - LiverToxDoxycycline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...