NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Dofetilide is an oral class III antiarrhythmic agent used for treatment and prevention of atrial fibrillation and flutter. Dofetilide has had limited clinical use, but has not been linked to an increased rate of serum enzyme elevations during therapy or to instances of clinically apparent liver injury.
Background
Dofetilide (doe fet' i lide) is a methanesulfonamide antiarrhythmic agent that is used to treat and prevent recurrence of atrial fibrillation and flutter. Dofetilide is considered a class III antiarrhythmic as it appears to act on a delayed rectifier of a specific potassium channel which results in prolongation of the recovery phase and delay in spread of the action potential. Dofetilide has been shown to lead to spontaneous conversion to normal sinus rhythm in up to 30% of patients with atrial fibrillation, compared to 1% of patients on placebo. Chronic therapy with dofetilide also increased the rate of maintenance of normal sinus rhythm after electro-cardioversion. Dofetilide was approved for use in the United States for atrial fibrillation and flutter in 1999, but its use is restricted and requires physician training in its use as well as initiation of therapy with 3 days of cardiac monitoring and assessment of renal function. Dofetilide is available in capsules of 125, 250 and 500 µg under the brand name Tikosyn. The recommended dose is 500 µg twice daily with dose modification based upon creatinine clearance. Common side effects include headache, dizziness, chest pain and nausea. Uncommon, but potentially severe adverse events include prolongation of the QTc interval (in up to 26%), torsades de pointes (a polymorphic form of ventricular tachycardia), ventricular fibrillation and sudden cardiac death. The frequency of these proarrhythmic side effects is largely dose related.
Hepatotoxicity
In clinical trials, serum aminotransferase and alkaline phosphatase elevations were no more common during dofetilide than placebo therapy. Some degree of ALT elevation was reported in 15% of dofetilide but a similar proportion of placebo recipients; these elevations were above 3 times the upper limit of normal in 1.5% vs 2.0%. Thus, the background rate of serum ALT elevations in patients with atrial fibrillation eligible for dofetilide treatment appears to be high. Despite this, dofetilide has not been linked to instances of clinically apparent liver injury with symptoms or jaundice. The product label for dofetilide does not mention hepatotoxicity and does not specifically recommend monitoring of liver tests.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which dofetilide might cause liver injury is unknown. Dofetilide has a sulfonamide-like structure, but has not been linked to a high rate of hypersensitivity ("sulfa") reactions. The absence of hepatic side effects may be attributable to the low doses used (1 mg or less daily). Dofetilide is metabolized in the liver predominantly by CYP 3A4 and it is susceptible to multiple drug-drug interactions. Dofetilide should not be used in combination with drugs that inhibit CYP 3A4 activity which can cause increases in drug levels and risks of serious ventricular arrhythmias.
Outcome and Management
There is little evidence that dofetilide can cause liver injury, but it has a narrow therapeutic-toxic ratio in relation to cardiac arrhythmias, prolongation of the QTc interval and risk for torsades de pointes. There is little information about cross sensitivity to liver injury between other oral antiarrhythmics and dofetilide.
Drug Class: Antiarrhythmic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dofetilide – Generic, Tikosyn®
DRUG CLASS
Antiarrhythmic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
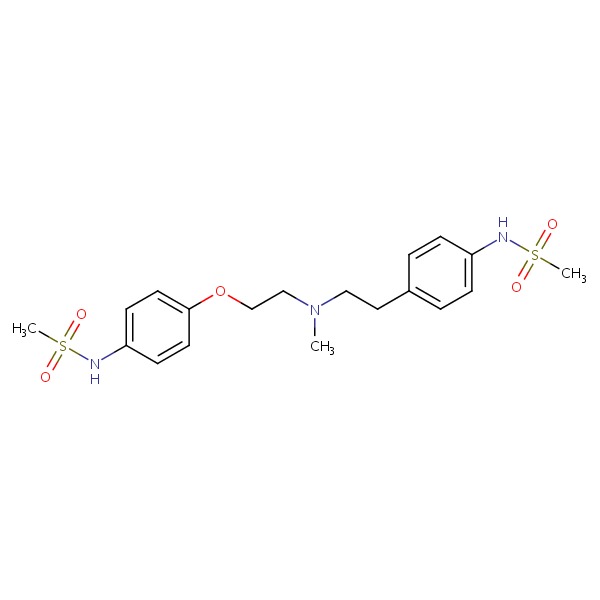
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dofetilide | 115256-11-6 | C19-H27-N3-O5-S2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 September 2016
- Zimmerman HJ. Antiarrhythmics. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 642-4.(Expert review of hepatotoxicity of antiarrhythmics published in 1999 before the availability of dofetilide).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of cardiovascular agents; does not discuss dofetilide).
- Sampson KJ, Kass RS. Antiarrhythmic drugs. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 815-48.(Textbook of pharmacology and therapeutics).
- Torp-Pedersen C, Møller M, Bloch-Thomsen PE, Køber L, Sandøe E, Egstrup K, Agner E, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. N Engl J Med 1999; 341: 857-65. [PubMed: 10486417](Among 1518 patients with congestive heart failure and left ventricular dysfunction treated with dofetilide or placebo for an average of 18 months, treatment was associated with a higher rate of conversion to normal sinus rhythm [12% vs 1%] and a decrease in need for re-hospitalization, but no difference in mortality; adverse event rates were similar in the 2 groups; no mention of ALT elevations or hepatotoxicity).
- Roden DM. Antiarrhythmic drugs: from mechanisms to clinical practice. Heart 2000; 84: 339-46. [PMC free article: PMC1760959] [PubMed: 10956304](Overview of antiarrhythmic drugs which are separated into four classes based upon molecular target: I being sodium channel blockers; II beta blockers; III potassium channel blockers; and, IV calcium channel blockers; some agents having multiple targets).
- Singh S, Zoble RG, Yellen L, Brodsky MA, Feld GK, Berk M, Billing CB Jr. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation 2000; 102: 2385-90. [PubMed: 11067793](Among 325 patients with atrial fibrillation-flutter treated with dofetilide [25, 250 or 500 µg] vs placebo twice daily, normal sinus rhythm conversion was more frequent with dofetilide [6-30%] than placebo [1%] and was more frequently maintained [37-58% vs 25%], but torsade de pointes occurred only with dofetilide [n=2; 0.8%] as did sudden cardiac death [n=1]; no mention of ALT elevations or hepatotoxicity).
- Grines CL. Safety and effectiveness of dofetilide for conversion of atrial fibrillation and nesiritide for acute decompensation of heart failure: a report from the cardiovascular and renal advisory panel of the Food and Drug Administration. Circulation. 2000; 101: E200-1. [PubMed: 10831533](Review of the safety and efficacy of dofetilide for atrial fibrillation by the FDA; mentions its narrow therapeutic window, need to adjust dose based upon creatinine clearance and multiple potential drug-drug interactions; no mention of ALT elevations or hepatotoxicity).
- Boriani G, Lubinski A, Capucci A, Niederle R, Kornacewicz-Jack Z, Wnuk-Wojnar AM, Borggrefe M, et al.; Ventricular Arrhythmias Dofetilide Investigators. A multicentre, double-blind randomized crossover comparative study on the efficacy and safety of dofetilide vs sotalol in patients with inducible sustained ventricular tachycardia and ischaemic heart disease. Eur Heart J 2001; 22: 2180-91. [PubMed: 11913480](Among 135 Italian patients with ischemic heart disease at risk of arrhythmias who were treated with dofetilide [500 µg] or sotalol [160 mg] twice daily, overall rates of adverse events were similar in the two groups; no mention of ALT elevations, hypersensitivity reactions or withdrawals for liver related events).
- Møller M, Torp-Pedersen CT, Køber L. Dofetilide in patients with congestive heart failure and left ventricular dysfunction: safety aspects and effect on atrial fibrillation. The Danish Investigators of Arrhythmia and Mortality on Dofetilide (DIAMOND) Study Group. Congest Heart Fail 2001; 7 (3): 146-50. [PubMed: 11828153](In a further analysis of the Danish controlled trial of dofetilide vs placebo in 1518 patients with congestive heart failure [Torp-Pederson 1999], the overall adverse event and mortality rates were similar in the 2 groups, but 25 cases of torsade de pointes ventricular tachycardia occurred with dofetilide [2 deaths] but none with placebo; no mention of ALT elevations or hepatotoxicity).
- McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med 2003; 139: 1018-33. [PubMed: 14678922](Systematic review of literature on drugs for atrial fibrillation; mentions that the major safety concern is the potential to induce polymorphic ventricular tachycardia [torsades de pointes], which usually occurs during the first 3 days of treatment and is most common with quinidine and dofetilide [up to 12%]).
- Guanzon AV, Crouch MA. Phase IV trial evaluating the effectiveness and safety of dofetilide. Ann Pharmacother 2004; 38: 1142-7. [PubMed: 15161945](Among 107 patients with atrial fibrillation or flutter treated with dofetilide in clinical practice, none developed torsades de pointes although 26% had excessive QTc prolongation, and rates of conversion to and maintenance of normal sinus rhythm were similar or higher than reported in registration trials; no mention of ALT elevations or hepatotoxicity).
- Drugs for cardiac arrhythmias. Treat Guidel Med Lett 2007; 5: 51-8. [PubMed: 17505408](Concise review of drugs for arrhythmias; dofetilide is used orally to convert atrial fibrillation and maintain sinus rhythm after cardioversion, but is available only through a restricted distribution program requiring that it be started in the hospital with cardiac monitoring).
- Camm J. Antiarrhythmic drugs for the maintenance of sinus rhythm: risks and benefits. Int J Cardiol 2012; 155: 362-71. [PubMed: 21708411](Review of the clinical efficacy and safety of drug therapy for maintenance of sinus rhythm after conversion from atrial fibrillation; mentions the proarrhythmic adverse effects of dofetilide and the frequency of torsades de pointes [0.3-4.7%]).
- Treatment of atrial fibrillation. Med Lett Drugs Ther 2014; 56 (1446): 53-8. [PubMed: 25046325](Review of drug therapy of atrial fibrillation; mentions that dofetilide is used for rhythm control, particularly in patients with left ventricular dysfunction, but requires in-hospital dose titration and can cause torsades de pointes).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 7 were attributed to antiarrhythmics including 5 to amiodarone, 2 to dronedarone, but none to dofetilide).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Intravenous dofetilide, a class III antiarrhythmic agent, for the termination of sustained atrial fibrillation or flutter. Intravenous Dofetilide Investigators.[J Am Coll Cardiol. 1997]Intravenous dofetilide, a class III antiarrhythmic agent, for the termination of sustained atrial fibrillation or flutter. Intravenous Dofetilide Investigators.Falk RH, Pollak A, Singh SN, Friedrich T. J Am Coll Cardiol. 1997 Feb; 29(2):385-90.
- Review Dofetilide: a review of its use in atrial fibrillation and atrial flutter.[Drugs. 1999]Review Dofetilide: a review of its use in atrial fibrillation and atrial flutter.McClellan KJ, Markham A. Drugs. 1999 Dec; 58(6):1043-59.
- Electrophysiologic effects of the new class III antiarrhythmic drug dofetilide compared to the class IA antiarrhythmic drug quinidine in experimental canine atrial flutter: role of dispersion of refractoriness in antiarrhythmic efficacy.[J Cardiovasc Electrophysiol. 1...]Electrophysiologic effects of the new class III antiarrhythmic drug dofetilide compared to the class IA antiarrhythmic drug quinidine in experimental canine atrial flutter: role of dispersion of refractoriness in antiarrhythmic efficacy.Cha Y, Wales A, Wolf P, Shahrokni S, Sawhney N, Feld GK. J Cardiovasc Electrophysiol. 1996 Sep; 7(9):809-27.
- Review Efficacy of class III antiarrhythmics and magnesium combination therapy for atrial fibrillation.[Pharm Pract (Granada). 2012]Review Efficacy of class III antiarrhythmics and magnesium combination therapy for atrial fibrillation.Wang A. Pharm Pract (Granada). 2012 Apr; 10(2):65-71. Epub 2012 Jun 30.
- Electrophysiologic Effects of the New Class III Antiarrhythmic Drug Dofetilide in an Experimental Canine Model of Pacing-induced Atrial Fibrillation.[J Cardiovasc Pharmacol Ther. 1...]Electrophysiologic Effects of the New Class III Antiarrhythmic Drug Dofetilide in an Experimental Canine Model of Pacing-induced Atrial Fibrillation.Feld GK, Cha Y. J Cardiovasc Pharmacol Ther. 1997 Jul; 2(3):195-203.
- Dofetilide - LiverToxDofetilide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...