NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Docetaxel is an antineoplastic agent that has a unique mechanism of action as an inhibitor of cellular mitosis and that currently plays a central role in the therapy of many solid tumors including breast and lung cancer. Docetaxel therapy is frequently associated with serum enzyme elevations which are usually transient and mild, but more importantly has been linked to rapid onset, severe hypersensitivity reactions that can be associated with acute hepatic necrosis, liver failure and death.
Background
Docetaxel (doe" se tax' el) is a complex diterpenoid molecule that contains a central 8-member taxane ring. Docetaxel is a semisynthetic analogue of paclitaxel and was initially isolated from the needles of the European Yew tree (Taxus baccata). It is a potent antineoplastic agent and its mechanism of action appears to be mediated by its binding to microtubulin, which is important in the mitotic phase of cell division. The binding of docetaxel prevents the disassembly of the cytoskeletal microtubules, preventing cell division and leading to cell death. Docetaxel was approved for use in the United States in 1996 and it remains an important agent in the therapy of several neoplasms including breast, gastric, prostate, head and neck, and non-small cell lung cancer. Docetaxel is available in solution for injection generically and under the brand names such as Taxotere and Docefrez. Docetaxel is administered intravenously, typically as one hour infusions every three weeks in combination with other antineoplastic agents. The dose varies by indication and body weight. Preexisting liver disease is considered a relative contraindication of its use. Side effects are common and include diarrhea, nausea, vomiting, mucositis, fatigue, myalgias, skin rash, alopecia, phlebitis, bone marrow suppression, fluid retention, cardiomyopathy, peripheral neuropathy and hypersensitivity reactions. Premedication with oral corticosteroids is recommended to prevent or at least ameliorate severe hypersensitivity reactions. The product label for docetaxel includes a black box warning of toxic deaths, hepatotoxicity, neutropenia, hypersensitivity reactions and fluid retention, with rates of death ranging from 0.6% to 2.8% of patients, the highest risk in those with preexisting liver test abnormalities.
Hepatotoxicity
Docetaxel has been associated with serum aminotransferase elevations in up to half of patients, but values greater than 5 times the upper limit of normal (ULN) occur in less than 2%. Similar rates of alkaline phosphatase elevations and occasional mild bilirubin elevations also occur. The abnormalities are usually asymptomatic, mild and self-limited, rarely requiring dose modification or discontinuation. Despite the frequency of serum enzyme elevations during therapy, clinically apparent liver injury from docetaxel is rare. Nevertheless, individual case reports of severe acute hepatic necrosis attributed to docetaxel have been published, usually arising within a few days or weeks after a severe hypersensitivity reaction to the first or second infusion of docetaxel (Case 1). The typical case arises within days of the infusion of docetaxel and is associated with rapid, marked rises in serum aminotransferase levels with subsequent appearance of jaundice. With severe injury there is early hepatic and multiorgan failure with jaundice and progressive hepatic encephalopathy, coagulopathy, and ascites. Immunoallergic features (fever, rash, flushing) are common initially, but may be obscured by corticosteroid therapy. Liver biopsy generally reveals zone 3 (centrolobular) necrosis and variable degrees of inflammation and cholestasis. Because docetaxel is often given with other antineoplastic agents, liver injury arising during therapy cannot always be attributed reliably to docetaxel as opposed to another specific agent. Furthermore, docetaxel in combination with other antineoplastic agents may be associated with reactivation of hepatitis B, increased risk of opportunistic viral infections, sinusoidal obstruction syndrome and sepsis, any of which can cause liver test abnormalities or clinically apparent liver injury.
Likelihood score: C (probable cause of acute hepatic necrosis associated with a hypersensitivity reaction to an infusion).
Mechanism of Injury
Docetaxel likely has a direct toxic effect on hepatocytes, accounting for the frequency of serum enzyme elevations during therapy, particularly with higher doses. Cases of clinically apparent liver injury have usually occurred in the setting of a severe hypersensitivity reaction, implying an immunoallergic component of injury. Docetaxel is metabolized in the liver by the cytochrome P450 system, predominantly CYP 3A4 and 3A5 and metabolites excreted in bile and feces.
Outcome and Management
Clinically significant liver injury from docetaxel is uncommon. Nevertheless, routine monitoring of liver tests is recommended before each docetaxel infusion, and administration held if serum bilirubin is elevated, ALT or AST are above 1.5 times ULN or alkaline phosphatase is above 2.5 times ULN. The serum enzyme elevations that occur on docetaxel therapy are usually self-limited and resolve with temporary discontinuation or modification of dosage. Liver injury associated with hypersensitivity reactions can be severe and docetaxel should be discontinued immediately. Re-exposure should be avoided. Because docetaxel is a structural analogue of paclitaxel, some degree of cross sensitivity to hypersensitivity reactions and hepatic injury should be expected. High doses of corticosteroids are often used to treat the hypersensitivity reaction, but it is not clear whether corticosteroids ameliorate the liver damage.
Drug Class: Antineoplastic Agents, Taxanes
Other Drugs in the Subclass, Taxanes: Cabazitaxel, Paclitaxel
CASE REPORT
Case 1. Acute hepatocellular injury after an infusion of docetaxel.(1)
A 31 year old woman with endometrial cancer developed abdominal pain and serum aminotransferase elevations the week following an initial infusion of docetaxel and carboplatin. She had no history of liver disease, drug allergies, alcohol abuse or risk factors for viral hepatitis. Her other medical conditions included seasonal allergies, reactive airway disease and dyspepsia. Her endometrial carcinoma was initially treated surgically with hysterectomy and bilateral oophorectomy, followed by pelvic irradiation and intravenous cisplatin which was poorly tolerated. She then started paclitaxel and carboplatin but had an immediate hypersensitivity reaction during the infusion manifested by chest tightness with arm and facial flushing despite premedication with antihistamines and dexamethasone. The following week her chemotherapy regimen was switched to docetaxel and carboplatin with intravenous dexamethasone followed by oral dexamethasone. She had mild symptoms of hypersensitivity during the infusions, but during the ensuing week she developed fatigue and abdominal pain. At the time of the next planned infusion, serum ALT was found to be 649 U/L, AST 211 U/L and alkaline phosphatase 161 U/L (R=11.7: hepatocellular). Serum bilirubin and albumin were normal and INR 0.9. (Table). Therapy was held. The following day, serum ALT levels had decreased. Serologic markers for acute hepatitis A, B and C were negative. Serum ANA was weakly positive (1:80) but SMA and AMA were negative. Imaging of the liver was normal without evidence of biliary or portal venous obstruction. Her symptoms resolved and over the next few weeks her serum aminotransferase levels fell to near normal levels. She was restarted on carboplatin without docetaxel and serum enzymes remained normal or minimally elevated.
Key Points
| Medication: | Docetaxel (124 mg iv once) |
|---|---|
| Pattern: | Hepatocellular (R=11.7) |
| Severity: | 1+ symptomatic (no jaundice) |
| Latency: | 1 week |
| Recovery: | 5 weeks |
| Other medications: | Carboplatin, dexamethasone |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre (-2 mo) | Pre | 25 | 73 | 0.2 | |
| Pre (-1 mo) | Pre | Paclitaxel and carboplatin: hypersensitivity reaction during infusion | |||
| Pre (-1 wk) | Pre | 35 | 66 | 0.3 | Docetaxel and carboplatin |
| 7 days | 0 | 649 | 161 | 0.2 | Therapy held |
| 8 days | 1 day | 545 | 148 | 0.3 | INR 0.9 |
| 14 days | 7 days | 295 | 92 | 0.3 | |
| 19 days | 12 days | 126 | 78 | 0.2 | Carboplatin restarted |
| 26 days | 19 days | 64 | 87 | 0.3 | |
| 35 days | 28 days | 94 | 95 | 0.3 | |
| 44 days | 37 days | 30 | 84 | 0.2 | |
| Normal Values | <40 | <125 | <1.2 | ||
Comment
This young woman with endometrial cancer and history of season allergies, had a mild hypersensitivity reaction during an infusion of paclitaxel and significant hepatic injury with a subsequent single infusion of docetaxel. The injury was symptomatic but without jaundice or evidence of hepatic synthetic dysfunction. The rapid rise and equally rapid fall in serum aminotransferase levels suggests direct hepatic injury and acute hepatic necrosis. In this case the injury was mild and self-limited injury, but docetaxel hypersensitivity reactions can be severe and associated with jaundice and hepatic failure. This patient appeared to have cross sensitivity with paclitaxel.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Docetaxel – Generic, Taxotere®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Docetaxel | 148408-66-6 | C43-H53-N-O14.3H2-O |
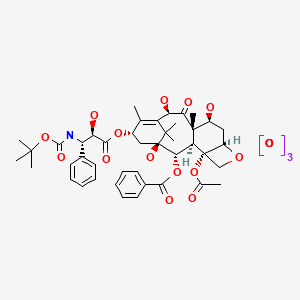
|
CITED REFERENCE
- 1.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
ANNOTATED BIBLIOGRAPHY
References updated: 13 October 2020
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp.673-708.(Textbook of hepatotoxicity published in 1999; docetaxel is not mentioned).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; the taxanes are not specifically discussed).
- Wellstein A, Giaccone G, Atkins MB, Sausille EA. Taxanes. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1187-9.(Textbook of pharmacology and therapeutics).
- Blaney SM, Seibel NL, O'Brien M, Reaman GH, Berg SL, Adamson PC, Poplack DG, et al. Phase I trial of docetaxel administered as a 1-hour infusion in children with refractory solid tumors: a collaborative pediatric branch, National Cancer Institute and Children's Cancer Group trial. J Clin Oncol. 1997;15:1538–43. [PubMed: 9193350](44 children with refractory solid tumors were given 103 courses of docetaxel every 21 days; major dose limiting toxicities were neutropenia, mild ALT elevations [<3 times ULN] occurred, but frequency and details were not given).
- Von Hoff DD. The taxoids: same roots, different drugs. Semin Oncol. 1997;24(4) Suppl 13:S13–3. [PubMed: 9335511](Review of the development of paclitaxel and docetaxel, clinical use, efficacy and toxicity stressing the differences between the two taxoids, which are due largely to differences in pharmacokinetics).
- Picus J, Schultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin Oncol. 1999;26(5) Suppl 17:14–8. [PubMed: 10604263](35 patients with prostate cancer were treated with docetaxel every 3 weeks [average of 6 cycles]; one had marked ALT elevations, but also had liver metastases).
- Crown J. A review of the efficacy and safety of docetaxel as monotherapy in metastatic breast cancer. Semin Oncol. 1999;26(1) Suppl 3:5–9. [PubMed: 10203264](Brief review of safety and efficacy of docetaxel in advanced breast cancer; no discussion of hepatotoxicity or ALT elevations).
- Burris HA 3rd. Single-agent docetaxel(Taxotere) in randomized phase III trials. Semin Oncol. 1999;26(3) Suppl 9:1–6. [PubMed: 10426452](Review of 3 randomized trials of docetaxel vs standard chemotherapy in women with breast cancer; adverse events were common, but hepatotoxicity was not mentioned).
- Fumoleau P. Efficacy and safety of docetaxel in clinical trials. Am J Health Syst Pharm. 1997;54(24) Suppl 2:S19–24. [PubMed: 9435929](Overall response rates to docetaxel and paclitaxel in advanced breast cancer ranged from 29-68% of patients; major dose related toxicities included neutropenia, mucositis, cardiomyopathy and fluid retention; hepatotoxicity was not mentioned).
- Alexandre J, Bleuzen P, Bonneterre J, Sutherland W, Misset JL, Guastalla J, Viens P, et al. Factors predicting for efficacy and safety of docetaxel in a compassionate-use cohort of 825 heavily pretreated advanced breast cancer patients. J Clin Oncol. 2000;18:562–73. [PubMed: 10653871](Among 825 patients with breast cancer undergoing therapy with docetaxel, the risk for febrile neutropenia was increased in those with liver dysfunction as defined by ALT or AST >1.5 and Alk P >3 times ULN [odds ratio 1.86]).
- Tomassini E, Muhizi J, al Raheb K, Steinbach G, Bemer M, Platini C. Presse Med. 2001;30:634. [Fulminant hepatocellular necrosis following administration of docetaxel] French. [PubMed: 11346902](52 year old woman with metastatic breast cancer developed jaundice and stupor 72 hours after an initial infusion of docetaxel [bilirubin 6.8 mg/dL, ALT 5540 U/L, GGT 116 U/L, protime 18 sec], with death from multiorgan failure and autopsy showing massive necrosis).
- Venturini M, Del Mastro L, Garrone O, Angiolini C, Merlano M, Bergaglio M, Tolino G, et al. Phase I, dose-finding study of capecitabine in combination with docetaxel and epirubicin as first-line chemotherapy for advanced breast cancer. Ann Oncol. 2002;13:546–52. [PubMed: 12056704](Among 23 women with advanced breast cancer treated with 139 cycles of docetaxel, capecitabine and epirubicin, 2 were withdrawn because of hepatic toxicity).
- Yeo W, Mok TS, Tse KK, Kwan WH, Lam KC, Ho WM, Chiu SK, et al. Phase II study of docetaxel and epirubicin in Chinese patients with metastatic breast cancer. Anticancer Drugs. 2002;13:655–62. [PubMed: 12172512](Among 46 patients treated with docetaxel and epirubicin, 6 [13%] developed abnormal ALT levels including two with hepatitis B reactivation, the 4 without hepatitis B being mild and transient).
- Sundar S, Chan SY. Cholestatic jaundice and pseudomembranous colitis following combination therapy with doxorubicin and docetaxel. Anticancer Drugs. 2003;14:327–9. [PubMed: 12679738](45 year old woman with advanced breast cancer developed febrile neutropenia after 2 cycles of docetaxel and doxorubicin with subsequent pseudo-membranous colitis and jaundice [peak bilirubin 5.3 mg/dL, ALT 46 U/L, Alk P 3436 U/L], resolving within 2 months).
- Pelegrí A, Calvo L, Mayordomo J I, Florián J, Vázquez S, Arcusa A, Martn-Richard M, et al. Spanish Group for Breast Cancer Research (GEICAM). Gemcitabine plus docetaxel administered every other week as first-line treatment of metastatic breast cancer: preliminary results from a phase II trial. Semin Oncol. 2004;31(2) Suppl 5:20–4. [PubMed: 15199528](Among 35 patients with metastatic breast cancer treated with up to 10 cycles of docetaxel and gemcitabine, 51% had aminotransferase elevations, which were >5 times ULN in 6%, but largely asymptomatic and self-limited).
- Goodin S, Medina P, Capanna T, Shih WJ, Abraham S, Winnie J, Doyle-Lindrud S, et al. Effect of docetaxel in patients with hormone-dependent prostate-specific antigen progression after local therapy for prostate cancer. J Clin Oncol. 2005;23:3352–7. [PubMed: 15738531](Among 25 patients with advanced prostate cancer given docetaxel in 3 week cycles, one had dose reduction for ALT elevations >5 times ULN).
- Kaira K, Takise A, Minato K, Iwasaki Y, Ishihara S, Takei Y, Tsuchiya S, et al. Phase II study of weekly docetaxel and cisplatin in patients with non-small cell lung cancer. Anticancer Drugs. 2005;16:455–60. [PubMed: 15746583](Among 40 patients with lung cancer treated with weekly docetaxel and cisplatin, 2 had ALT elevations >5 times ULN leading to dose reduction in 1).
- Cao Y, Wang ZQ, Guo Y, Feng FY, Hu XH, Xiong JP, Tang GD, et al. Ai Zheng. 2006;25:999–1002. [A randomized control clinical trial of Euruikang (docetaxel) in treatment of advanced non-small cell lung cancer (NSCLC)] Chinese. [PubMed: 16965682]
- Kasahara K, Kimura H, Shibata K, Araya T, Sone T, Oribe Y, Furusho S, et al. A phase II study of combination chemotherapy with docetaxel and carboplatin for patients with advanced or metastatic non-small cell lung cancer. Anticancer Res. 2006;26:3723–8. [PubMed: 17094391](Among 40 patients with advanced lung cancer treated with docetaxel and carboplatin, 6 [15%] had ALT elevations which were above 5 times ULN in one).
- Liu DG, Peng RJ, Feng FY, Hu XH, Tang GD, Xiong JP, Zhao HY, et al. Ai Zheng. 2006;25:1557–60. [Randomized controlled trial of two kinds of home-produced docetaxel in China for advanced breast cancer] Chinese. [PubMed: 17166386](Comparison of two formulations of docetaxel in 67 patients with advanced breast cancer found that transient ALT and AST elevations were common).
- Sinibaldi VJ, Elza-Brown K, Schmidt J, Eisenberger MA, Rosenbaum E, Denmeade SR, Pili R, et al. Phase II evaluation of docetaxel plus exisulind in patients with androgen independent prostate carcinoma. Am J Clin Oncol. 2006;29:395–8. [PubMed: 16891869](Among 14 patients with prostate cancer treated with docetaxel and exisulind, 21% had aminotransferase elevations above 5 times ULN).
- Aoki H, Ishidoya S, Ito A, Endoh M, Shimazui T, Arai Y. Experience of the treatment with gemcitabine, docetaxel, and carboplatin(GDC) chemotherapy for patients with small-cell carcinoma of the prostate. Int J Urol. 2006;13:1254–8. [PubMed: 16984566](Two patients with advanced prostate cancer were treated with docetaxel in combination with gemcitabine and carboplatin; one developed acute liver failure several months after a fifth cycle which was attributed to herpes zoster).
- Ohlmann CH, Kohlmorgen S, Sahi D, Engelmann U, Heidenreich A. Urologe A. 2007;46:1425–7. [Lethal course after chemotherapy with docetaxel. Acute liver failure with accompanying erythema multiforme major] German. [PubMed: 17563866](67 year old man with prostate cancer developed Stevens-Johnson Syndrome, neutropenia and thrombocytopenia after 5 weekly infusions of docetaxel, with subsequent rise in liver tests and jaundice that progressed to hepatic failure and death; few details provided).
- Chen JP, Lo Y, Yu CJ, Hsu C, Shih JY, Yang CH. Predictors of toxicity of weekly docetaxel in chemotherapy-treated non-small cell lung cancers. Lung Cancer. 2008;60:92–7. [PubMed: 17936403](Retrospective analysis of toxicity of 455 cycles of docetaxel in 155 patients with non-small cell lung cancer; 67 patients were withdrawn for toxicity, 2 were liver related; preexisting hepatitis B and C were risk factors for toxicity).
- James J, Murry DJ, Treston AM, Storniolo AM, Sledge GW, Sidor C, Miller KD. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs. 2007;25:41–8. [PubMed: 16969706](Among 15 women with advanced breast cancer treated with docetaxel and 2-methoxyestradiol, 3 [20%] had ALT elevations, which resolved with dose reductions in 2 and stopping docetaxel in 1).
- Minami H, Kawada K, Sasaki Y, Tahara M, Igarashi T, Itoh K, Fujii H, et al. Population pharmacokinetics of docetaxel in patients with hepatic dysfunction treated in an oncology practice. Cancer Sci. 2009;100:144–9. [PubMed: 19018756](Analysis of pharmacokinetics of docetaxel in 200 patients found that liver test abnormalities were associated with delayed clearance and recommended 20-40% reduction in dose).
- Kobayashi K, Yokonishi T, Ito Y, Matsumoto T, Umemoto S, Osaka K, Nakamura M, et al. Hinyokika Kiyo. 2010;56:203–7. [Low-dose docetaxel, estramustine and dexamethasone combination chemotherapy for hormone-refractory prostate cancer] Japanese. [PubMed: 20448443](Among 62 patients with advanced prostate cancer treated with docetaxel, estramustine and dexamethasone for an average of 11 cycles, only 1 had transient ALT elevation >5 times ULN).
- Wang Z, Liang X, Yu J, Zheng X, Zhu Y, Yan Y, Dong N, et al. Non-genetic risk factors and predicting efficacy for docetaxel--drug-induced liver injury among metastatic breast cancer patients. J Gastroenterol Hepatol. 2012;27:1348–52. [PubMed: 22432938](Among 647 women with breast cancer treated with docetaxel, 67 [10%] developed liver injury [27% with jaundice], with a median time to onset of 14 days; risk factors were menopausal status, hepatitis B markers, and presence of liver metastases).
- Liang X, Zhang J, Zhu Y, Lu Y, Zhou X, Wang Z, Yu J, et al. Specific genetic polymorphisms of IL10-592 AA and IL10-819 TT genotypes lead to the key role for inducing docetaxel-induced liver injury in breast cancer patients. Clin Transl Oncol. 2013;15:331–4. [PubMed: 23143946](Among 129 patients with metastatic breast cancer treated with docetaxel, there were significant associations between specific polymorphisms of IL10, but not tumor necrosis factor and 40 instances of acute liver injury; although the associations were slight).
- Mandaliya H, Baghi P, Prawira A, George MK. A rare case of paclitaxel and/or trastuzumab induced acute hepatic necrosis. Case Rep Oncol Med. 2015;2015:825603. [PMC free article: PMC4641929] [PubMed: 26605100](62 year old woman with metastatic breast cancer received 4 cycles of doxorubicin and cyclophosphamide and developed pulmonary edema and respiratory failure within 12 hours of an initial infusion of paclitaxel and trastuzumab, dying one day later and autopsy showing acute hepatic necrosis; no liver test results provided).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] were attributed to antineoplastic agents of which only 1 was due to a taxane [docetaxel] Case 1).
- Oudard S, Fizazi K, Sengeløv L, Daugaard G, Saad F, Hansen S, Hjälm-Eriksson M, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017;35:3189–97. [PubMed: 28753384](Among 1,168 patients with metastatic prostate cancer treated with cabazitaxel or docetaxel, overall survival rates were similar while adverse events were higher in a higher dose arm of cabazitaxel; ALT elevations and hepatotoxicity were not mentioned).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Taxanes.[LiverTox: Clinical and Researc...]Review Taxanes.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Cabazitaxel.[LiverTox: Clinical and Researc...]Review Cabazitaxel.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Paclitaxel.[LiverTox: Clinical and Researc...]Review Paclitaxel.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Molecular profiling of docetaxel cytotoxicity in breast cancer cells: uncoupling of aberrant mitosis and apoptosis.[Oncogene. 2007]Molecular profiling of docetaxel cytotoxicity in breast cancer cells: uncoupling of aberrant mitosis and apoptosis.Hernández-Vargas H, Palacios J, Moreno-Bueno G. Oncogene. 2007 May 3; 26(20):2902-13. Epub 2006 Nov 13.
- Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells.[Mol Cancer Ther. 2005]Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells.Morse DL, Gray H, Payne CM, Gillies RJ. Mol Cancer Ther. 2005 Oct; 4(10):1495-504.
- Docetaxel - LiverToxDocetaxel - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...