NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Diazepam is a benzodiazepine that is available for both oral and intravenous administration; oral diazepam is used predominantly as an anxiolytic agent, while the intravenous form is used as an anticonvulsant. Use of intravenous diazepam has not been linked to serum enzyme elevations during therapy or to clinically apparent liver injury. In contrast, use of the oral form of diazepam has been linked to rare instances of cholestatic liver injury.
Background
Diazepam (dye az' e pam) is a benzodiazepine with particularly potent activity against spread of seizure activity in several animal models. The antiseizure activity of the benzodiazepines is mediated by their ability to enhance gamma-aminobutryic acid (GABA) mediated inhibition of synaptic transmission through binding to the GABA A receptor. The use of diazepam as an anticonvulsant is limited largely to intravenous therapy of status epilepticus. Oral diazepam is not as effective or well tolerated as a therapy for epilepsy as are other benzodiazepines such as clobazam, clonazepam and clorazepate. Diazepam was approved in the United States in 1963 and it is currently widely used to treat acute seizures and status epilepticus. Indications also include premedication before surgical operations and as conscious sedation for minor invasive procedures. Several generic forms of parenteral diazepam are available in ampules of 5 mg/mL. The typical recommended dose for status epilepticus is 5 to 10 mg given intravenously, which can be repeated at 10 to 15 minute intervals until control of seizure activity or to a maximum of 30 mg. Intramuscular administration can be used for premedication before general anesthesia. Common effects of the use of parenteral diazepam include somnolence, confusion, dysarthria, diplopia and coma. Acute overdose of diazepam can cause respiratory arrest and death.
Hepatotoxicity
Diazepam, as with other benzodiazepines, is rarely associated with serum ALT elevations, and clinically apparent liver injury from diazepam is extremely rare. In particular, parenterally administered diazepam has not been reported to cause serum enzyme elevations, even with prolonged use as with therapy of tetanus. Furthermore, there have been no convincing case reports of acute liver injury attributable to intravenous diazepam, although there are several case reports of acute liver injury from oral formulations. The onset of injury after oral use ranged from 4 to 12 weeks, and the typical pattern of serum enzyme elevations was cholestatic or mixed. Fever and rash have not been described nor has autoantibody formation. There have been no case reports of diazepam hepatotoxicity since the 1980's.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The liver injury from benzodiazepines is probably due to a rarely produced intermediate metabolite.
Outcome and Management
Patients with hepatic injury due to oral diazepam in the literature recovered completely without evidence of residual or chronic injury. No cases of acute liver failure or chronic liver injury due to diazepam have been described. There is no information about cross reactivity with other benzodiazepines (clobazam, clorazepate, lorazepam or alprazolam), but some degree of cross sensitivity should be assumed.
Drug Class: Anticonvulsants, see also Diazepam (Oral)
CASE REPORT
Case 1. Cholestatic hepatitis due to oral diazepam.
[Modified from: Fors B, Nilsson F. [Hepatitis probably induced by diazepam medication] Lakartidningen 1968; 65: 4528-31. Swedish. PubMed Citation]
A 33 year old woman was started on diazepam (2 mg three times daily) for anxiety and four months later developed symptoms of abdominal pain and jaundice. Serum ALT was 306 U/L, alkaline phosphatase was twice elevated and bilirubin was 4.2 mg/dL. A cholecystectomy was done for suspected cholelithiasis, but the gallbladder and biliary tree were normal. A liver biopsy showed acute hepatocellular injury and cholestasis compatible with drug induced liver disease. Diazepam was stopped and she recovered rapidly and had normal liver tests one month later. Tests for hepatitis A, B and C were not available.
Key Points
| Medication: | Diazepam (2 mg orally three times daily) |
|---|---|
| Pattern: | Mixed (R=~4) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 4 months |
| Recovery: | Complete recovery within a month after stopping |
| Other medications: | Birth control pills |
Comment
The patient developed an acute hepatitis-like illness four months after starting diazepam. A liver biopsy showed changes typical of drug induced liver disease and the pattern of enzyme elevations and biopsy did not suggest viral hepatitis, serological testing for which was not available at the time. Causality assessment can only rank this example as a “probable” case of diazepam induced liver injury. In view of the wide scale use of diazepam, instances of hepatic injury are very rare, and no cases of severe, prolonged, persistent or fatal liver injury from diazepam have been published.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Diazepam – Valium®
DRUG CLASS
Anticonvulsants
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Diazepam | 439-14-5 | C16-H13-Cl-N2-O |
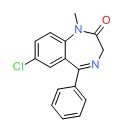 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 January 2017
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 498-516.(Expert review of benzodiazepines and liver injury published in 1999; mentions rare instances of cholestatic hepatitis have been reported due to alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam and hepatocellular injury with clorazepate and clotiazepam, but no reports of hepatic injury with lorazepam, prazepam, oxazepam and temazepam).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007: pp 485-506.(Review of anticonvulsant induced liver injury published in 2007; the benzodiazepines are not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006, pp. 501-525.(Textbook of pharmacology and therapeutics).
- Cook GC, Sherlock S. Jaundice and its relation to therapeutic agents. Lancet 1965; 1: 175-9. PubMed Citation (11 cases of drug induced liver disease, all due to agents no longer in use; 2 patients were receiving diazepam, but other agents were more likely the cause).
- Cunningham ML. Acute hepatic necrosis following treatment with amitriptyline and diazepam. Brit J Psychiat 1965; 111: 1107-9. [PubMed: 5841222](54 year old woman with dementia-depression placed on amitriptyline and diazepam developed progressive confusion 5 months later followed by jaundice, obtundation and death [bilirubin 5.2 mg/dL, ALT 200 U/L, Alk P 9.5 unknown units], autopsy showed small liver but no description of histology).
- Buchanan N, Cane RD. Liver function tests and the prolonged use of high-dose diazepam with special reference to tetanus. S Afr Med J 1978; 54: 768. [PubMed: 741305](After noting jaundice in a few patients receiving high doses of diazepam for tetanus, the authors prospectively tested 4 patients finding no consistent rises in serum ALT, Alk P or bilirubin on therapy).
- Stacher G. Intrahepatic cholostasis following combined diazepam-barbiturate therapy in patients with tetanus]. Wien Klin Wochenschr. 1973; 85: 401-6. German. [PubMed: 4705881]
- Franks E, Jacobs WH. Cholestatic jaundice possibly due to benzodiazepine-type drugs. Mo Med 1975; 72: 605-6. (40 year old woman on multiple drugs including chlorpromazine developed jaundice [bilirubin 2.0 rising to 9.7 mg/dL, ALT 280 U/L, Alk P 546 U/L, 16% eosinophils], seemed to worsen on benzodiazepines including chlordiazepoxide, diazepam and flurazepam, resolving rapidly when they were stopped, but attribution to benzodiazepines difficult .).
- Fors B, Nilsson F. [Hepatitis probably induced by diazepam medication] Lakartidningen 1968; 65: 4528-31. Swedish. [PubMed: 5745628](24 and 34 year old women developed mild hepatitis with jaundice 1 and 4 months after starting diazepam [bilirubin 11 and 4.2 mg/dL, ALT 434 U/L Alk ~2 times ULN], rapid resolution with stopping).
- Fang MH, Ginsberg AL, Dobbins WO 3rd. Cholestatic jaundice associated with flurazepam hydrochloride. Ann Intern Med 1978; 89: 363-4. [PubMed: 28685](70 year old man developed fatigue followed by pruritis 3 months after starting flurazepam [bilirubin 6.6 mg/dL, ALT 179 U/L, Alk P 232 U/L, no eosinophilia], improving only when drug withdrawn).
- Bonkovsky HL, Sinclair PR, Emery S, Sinclair JF. Seizure management in acute hepatic porphyria: risks of valproate and clonazepam. Neurology 1980; 30: 588-92. [PubMed: 6770287](38 year old man with acute intermittent porphyria and seizures did not respond to clonazepam, and testing in chicken embryos showed that it increased hepatic prophyrins and ALA synthase activity).
- Reynolds R, Lloyd DA, Slinger RP. Cholestatic jaundice induced by flurazepam hydrochloride. Can Med Assoc J 1981; 124: 893-4. [PMC free article: PMC1705357] [PubMed: 7214289](44 year old woman taking flurazepam intermittently for 6 months developed abdominal pain [bilirubin 8.2 mg/dL, AST 106 U/L, Alk P 209 U/L], resolving rapidly upon stopping, biopsy showed intrahepatic cholestasis).
- Tedesco FJ, Mills LR. Diazepam(Valium) hepatitis. Dig Dis Sci 1982; 27: 470-2. [PubMed: 7075434](45 year old man developed elevations of ALT [130 U/L] while on isoniazid [for 1 day] and diazepam [3 days], which resolved upon stopping and recurred upon rechallenge with diazepam, but not isoniazid which was tolerated long term).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol 1982; 17: 205-11. [PubMed: 6982502](Among 572 cases of hepatotoxicity reported to a Danish registry between 1968 and 1978, 97 were due to psychotropic agents, but only two attributed to benzodiazepines).
- Roy-Byrne P, Vittone BJ, Uhde TW. Alprazolam-related hepatotoxicity. Lancet 1983; 2: 786-7. [PubMed: 6137615](30 year old woman was found to have elevated ALT [96 U/L] 2 weeks after starting alprazolam, which promptly resolved when drug was stopped; rechallenge led to asymptomatic ALT rise within 9 days [28 to 70 U/L]).
- Davion T, Capron-Chivrac D, Andrejak M, Capron JP. [Hepatitis due to antiepileptic agents] Gastroenterol Clin Biol 1985; 9: 117-26. [PubMed: 3920108](Review of hepatotoxicity of anticonvulsants; among benzodiazepines, cases of cholestatic hepatitis have been linked to chlordiazepoxide and diazepam, but liver injury from this class of drugs is rare).
- Judd FK, Norman TR, Marriott PF, Burrows GD. A case of alprazolam-related hepatitis. Am J Psychiatry 1986; 143: 388-9. [PubMed: 2869702](59 year old woman developed lethargy 2 weeks after starting alprazolam followed by jaundice [bilirubin 2.6 mg/dL, AST 156 U/L, Alk P 241 U/L], resolving within 4 weeks of switching to diazepam).
- Olsson R, Zettergren L. Anticonvulsant-induced liver damage. Am J Gastroenterol 1988; 83: 576-7. [PubMed: 3364416](30 year old man developed fever, rash and jaundice 6 weeks after starting phenytoin; he was switched to carbamazepine and clonazepine, but redeveloped jaundice 3 months later; resolved with stopping, but recurred with restarting clonazepine alone [bilirubin 1.8 mg/dL, ALT 1380 U/L, Alk P 176 U/L] without rash, fever or eosinophilia, resolving in 6 weeks of stopping clonazepine).
- Habersetzer F, Larrey D, Babany G, Degott C, Corbic M, Pessayre D, Benhamou J-P. Clotiazepam-induced acute hepatitis. J Hepatol 1989; 9: 256-9. [PubMed: 2572625](65 year old woman developed jaundice 6 months after starting clotiazepam [bilirubin 5.1 mg/dL, ALT 1028 U/L, GGT 143 U/L] with no rash or fever, resolving on stopping clotiazepam and no recurrence after taking triazolam and temazepam).
- Nahata MC, Murray RD, Zingarelli J, Li BU, McClung HJ, Lininger B. Efficacy and safety of a diazepam and meperidine combination for pediatric gastrointestinal procedures. J Pediatr Gastroenterol Nutr 1990; 10: 335-8. [PubMed: 2324894](Safety of use of intravenous diazepam in children undergoing endoscopy; no mention of ALT abnormalities or liver injury).
- Suzuki A, Aso K, Ariyoshi C, Ishimaru M. Acute intermittent porphyria and epilepsy: safety of clonazepam. Epilepsia 1992; 33: 108-11. [PubMed: 1733741](13 year old girl with acute intermittent porphyria worsened on valproate and on phenytoin therapy, but clonazepam led to control of seizures and no worsening of porphyria).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf 1996; 15: 378-93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine; “No child taking… benzodiazepines had raised liver enzyme levels,”).
- Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis 1999; 3: 433-64. [PubMed: 11291233](Review of drug induced cholestatic syndromes, listing many causes including chlordiazepoxide and flurazepam: “Benzodiazepines may cause cholestatic injury, although this is rare.”).
- Selim K, Kaplowitz N. Hepatotoxicity of psychotropic drugs. Hepatology 1999; 29: 1347-51. [PubMed: 10216114](Review of hepatotoxicity of phenothiazines, butyrophenones, tricyclics, MAO inhibitors, acetylcholesterase inhibitors, and psychotropic drugs of abuse; “benzodiazepines…have a very low hepatotoxic potential, with only case reports in the literature, usually with a cholestatic pattern”).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to benzodiazepines or other anticonvulsants).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 1 was attributed to phenytoin, 1 to valproate and 1 to carbamazepine, but none to benzodiazepines or other anticonvulsants).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther 2007; 25:1401-9. PubMed Citation (Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 20 were attributed to benzodiazepines including 5 for clorazepate, 5 alprazolam, 6 lorazepam and 4 diazepam, but compared to controls, relative risk of injury was increased only for clorazepate [8.3: estimated frequency 3.4 per 100,000 person-year exposures]).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scan 2008; 118: 281-90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants focusing upon phenytoin, valproate, carbamazepine; “Furthermore, hepatotoxicity has not been convincingly shown to be associated with the use of benzodiazepines”).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to benzodiazepines).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, benzodiazepines were not among the top 40 agents implicated).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to a benzodiazepine).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to a benzodiazepine).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; no mention of hepatotoxicity of benzodiazepines).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none to benzodiazepine anticonvulsants).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Diazepam (Oral).[LiverTox: Clinical and Researc...]Review Diazepam (Oral).. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children.[Cochrane Database Syst Rev. 2018]Review Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children.McTague A, Martland T, Appleton R. Cochrane Database Syst Rev. 2018 Jan 10; 1(1):CD001905. Epub 2018 Jan 10.
- Effects of the non-NMDA antagonists NBQX and the 2,3-benzodiazepine GYKI 52466 on different seizure types in mice: comparison with diazepam and interactions with flumazenil.[Br J Pharmacol. 1994]Effects of the non-NMDA antagonists NBQX and the 2,3-benzodiazepine GYKI 52466 on different seizure types in mice: comparison with diazepam and interactions with flumazenil.Löscher W, Hönack D. Br J Pharmacol. 1994 Dec; 113(4):1349-57.
- Review Anticonvulsant therapy for status epilepticus.[Cochrane Database Syst Rev. 2014]Review Anticonvulsant therapy for status epilepticus.Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Cochrane Database Syst Rev. 2014 Sep 10; 2014(9):CD003723. Epub 2014 Sep 10.
- Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies.[J Pharmacol Exp Ther. 1992]Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies.Facklam M, Schoch P, Bonetti EP, Jenck F, Martin JR, Moreau JL, Haefely WE. J Pharmacol Exp Ther. 1992 Jun; 261(3):1113-21.
- Diazepam (Intravenous) - LiverToxDiazepam (Intravenous) - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...