NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cyproheptadine is a first generation antihistamine used in the treatment of allergic rhinitis and urticaria and as an appetite stimulant. Cyproheptadine has been linked to rare instances of clinically apparent liver injury.
Background
Cyproheptadine (sye" proe hep' da deen) is a first generation antihistamine which also has anticholinergic and antiserotonergic activities that is used to treat allergic conditions including seasonal rhinitis, conjunctivitis, dermatitits and urticaria. Cyproheptadine belongs to the piperidine class of antihistamines. Its antiserotonergic properties have led to its use to treat side effects of the serotonin reuptake inhibitors and the serotonin syndrome. Cyproheptadine has also been used off-label as an appetite stimulant and to treat cyclic vomiting. Cyproheptadine is available by prescription only. Formulations include tablets, oral solutions and syrups in multiple generic forms and under the trade name Periactin. The usual adult oral dose is 4 mg three to four times daily. Common side effects include excessive sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and precipitate acute narrow angle glaucoma.
Hepatotoxicity
Unlike most first generation antihistamines, cyproheptadine has been associated with several instances of clinically apparent liver injury. The few cases that have been described had a time to onset of 1 to 6 weeks and a cholestatic or mixed pattern of liver enzyme elevations. Immunoallergic and autoimmune features were not present and most patients recovered rapidly without residual. Acute liver failure due to cyproheptadine has not been described.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of liver injury due to cyproheptadine is not known, but is clearly idiosyncratic and perhaps due to its hepatic metabolism to a toxic intermediate.
Outcome and Management
Most instances of acute liver injury due to cyproheptadine have been mild-to-moderate in severity and self-limited in course and outcome. In one case report, there was recurrence of injury when cyproheptadine was restarted. There is no information on cross sensitivity to hepatic injury among the various antihistamines, but use of an antihistamine of another class after clinically apparent liver injury due to cyproheptadine is likely to be safe.
References on the safety and potential hepatotoxicity of antihistamines are given together after the Overview section on Antihistamines.
Drug Class: Antihistamines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cyproheptadine – Generic, Periactin®
DRUG CLASS
Antihistamines
Product labeling at DailyMed, National Library of Medicine, NIH
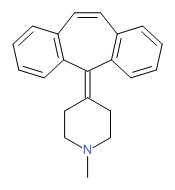
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cyproheptadine | 129-03-3 | C21-H21-N |
 |
- Review Rupatadine: global safety evaluation in allergic rhinitis and urticaria.[Expert Opin Drug Saf. 2016]Review Rupatadine: global safety evaluation in allergic rhinitis and urticaria.González-Núñez V, Bachert C, Mullol J. Expert Opin Drug Saf. 2016 Oct; 15(10):1439-48. Epub 2016 Aug 19.
- Review Rupatadine in allergic rhinitis and chronic urticaria.[Allergy. 2008]Review Rupatadine in allergic rhinitis and chronic urticaria.Mullol J, Bousquet J, Bachert C, Canonica WG, Gimenez-Arnau A, Kowalski ML, Martí-Guadaño E, Maurer M, Picado C, Scadding G, et al. Allergy. 2008 Apr; 63 Suppl 87:5-28.
- Comparison of a new antihistamine HC 20-511 with cyproheptadine (Periactin) in chronic urticaria.[Acta Allergol. 1977]Comparison of a new antihistamine HC 20-511 with cyproheptadine (Periactin) in chronic urticaria.Kuokkanen K. Acta Allergol. 1977 Oct; 32(5):316-20.
- Cold urticaria in Thai children: comparison between cyproheptadine and ketotifen in the treatment.[Asian Pac J Allergy Immunol. 1...]Cold urticaria in Thai children: comparison between cyproheptadine and ketotifen in the treatment.Visitsunthorn N, Tuchinda M, Vichyanond P. Asian Pac J Allergy Immunol. 1995 Jun; 13(1):29-35.
- Comparison of the new antihistamine acrivastine (BW 825C) versus cyproheptadine in the treatment of idiopathic cold urticaria.[Dermatologica. 1988]Comparison of the new antihistamine acrivastine (BW 825C) versus cyproheptadine in the treatment of idiopathic cold urticaria.Neittaanmäki H, Fräki JE, Gibson JR. Dermatologica. 1988; 177(2):98-103.
- Cyproheptadine - LiverToxCyproheptadine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...