NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Copanlisib is an intravenously administered phosphatidylinositol-3 kinase inhibitor that is used to treat relapsed and refractory follicular lymphoma. Copanlisib is associated with a high rate of minor serum enzyme elevations during therapy and has been reported to cause clinically apparent acute liver injury that can be severe and even fatal.
Background
Copanlisib (koe" pan lis' ib) is an intravenously administered small molecule inhibitor of phosphatidylinositol 3-kinase (PI3K) alpha and delta which are essential components in B cell signaling pathways that drive survival of B cells and their migration to lymph nodes and bone marrow. Inhibition of this pathway inhibits B cell chemotaxis and adherence and reduces cell viability. This pathway is upregulated in many B cell malignancies and is known to be critical for proliferation and survival of leukemia and lymphomatous malignant B lymphocytes. Copanlisib has been shown to result in high rates of objective response in patients with refractory or relapsed follicular lymphoma and was given accelerated approval for this use in the United States in 2017. Copanlisib is available in as lyophilized powder in 60 mg single dose vials under the brand name Aliqopa. The recommended dose is 60 mg given as an intravenous infusion on days 1, 8 and 15 of 28-day cycles. Side effects are common but usually mild-to-moderate in severity, and include nausea, diarrhea, headache, stomatitis, fever, pain, rash, infections, arthralgia and fatigue. Common laboratory abnormalities can include hyperglycemia and cytopenias. Severe adverse events can include marked hypertension, severe diarrhea, neutropenia, infectious and non-infectious pneumonitis, severe cutaneous reactions and embryo-fetal toxicity.
Hepatotoxicity
In clinical trials of copanlisib the rates of serum enzyme elevations during therapy ranged from 23% to 25% but were above 5 times the ULN in only 1% to 2%. Instances of clinically apparent liver injury were not reported in prelicensure trials of copanlisib, but the total number of patients exposed was limited. Since its approval and more general use, there have been no published reports of liver injury with jaundice associated with copanlisib therapy. In contrast, idelalisib, another small molecule inhibitor of PI3K, has been linked to instances of clinically apparent acute liver injury some of which have been fatal.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The reason why copanlisib causes serum enzyme elevations is not known, but may relate to direct toxicity to hepatocytes caused by inhibition of PI3K activity, which plays a central role in many metabolic pathways. Copanlisib is metabolized primarily by the cytochrome P450 system and it is susceptible to drug-drug interactions with strong inducers or inhibitors of CPY 3A.
Outcome and Management
Serum enzyme elevations are not uncommon during cancer chemotherapy with copanlisib and may occasionally be dose limiting. Monitoring of liver tests is recommended in patients receiving copanlisib. Copanlisib should be temporarily discontinued if ALT or AST values rise above 5 times the ULN and treatment resumed only if and when values improve significantly and then with a careful monitoring. Elevations of aminotransferase values of more than 20 times the ULN, or appearance of jaundice or symptoms of liver injury should trigger permanent discontinuation. There is no published information on cross sensitivity to hepatic injury among the different PI3K kinase inhibitors.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Copanlisib – Aliqopa®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
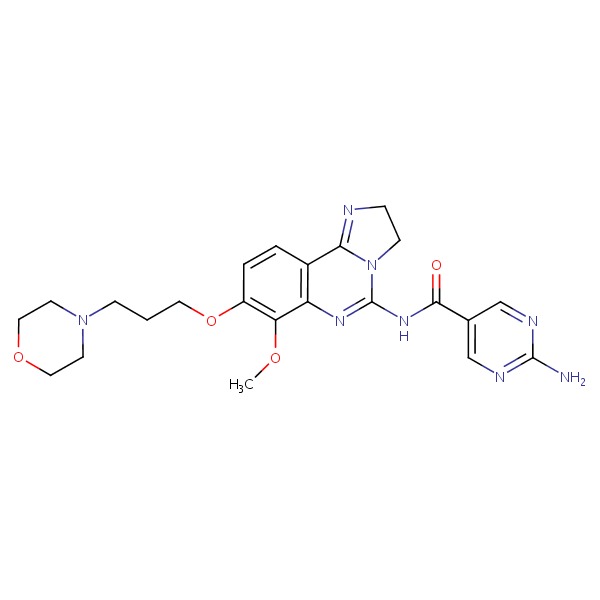
| Copanlisib | 1032568-63-0 | C26-H23-N7-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2018
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Kinase inhibitors. Gefitinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents; does not discuss copanlisib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy). - Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370: 997-1007. [PMC free article: PMC4161365] [PubMed: 24450857](Among 220 patients with relapsed CLL treated in a placebo controlled trial, progression free survival improved with idelalisib and rituximab compared to rituximab alone, but side effects were more common with the combination including ALT or AST elevations [35% vs 19%], which were above 5 times ULN in 5% vs 1% and led to drug discontinuations in some patients, but there were no clinically apparent cases of liver injury).
- Patnaik A, Appleman LJ, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Weiss GJ. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Ann Oncol 2016; 27: 1928-40. [PMC free article: PMC5035790] [PubMed: 27672108](Dose ranging studies in patients with diabetes and patients with various forms of cancer identified the maximum tolerated dose and yielded promising results in follicular lymphoma; adverse events were frequent but generally mild-to-moderate in severity, transient and asymptomatic ALT elevations occurring in 34% of subjects but only one had levels above 5 times ULN).
- Greenwell IB, Ip A, Cohen JB. PI3K inhibitors: understanding toxicity mechanisms and management. Oncology (Williston Park) 2017; 31: 821-8. [PubMed: 29179250](Review of the toxicities of PI3K inhibitors, their pathogenesis, frequency and management; mentions that idelalisib is associated with autoimmune hepatitis-like reactions that generally respond to drug discontinuation and that liver injury is less frequent and milder with copanlisib therapy).
- Markham A. Copanlisib: first global approval. Drugs 2017; 77: 2057-62. [PubMed: 29127587](Review of the mechanism of action, history of development, clinical efficacy and safety of copanlisib does not mention ALT elevations or hepatotoxicity).
- Dreyling M, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, Kim WS, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol 2017; 35: 3898-905. [PubMed: 28976790](Among 142 patients with relapsed or refractory indolent lymphoma treated with copanlisib, the objective response rate was 59% and adverse events were frequent including ALT elevations in 23% which were above 5 times ULN in 2 patients [2%]).
- Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, Verhoef G, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol 2017; 28: 2169-78. [PMC free article: PMC5834070] [PubMed: 28633365](Among 84 patients with lymphoma treated with copanlisib on days 1, 8 and 15 of 28-day cycles, the objective response rate was 44% in patients with indolent lymphoma and 27% with aggressive lymphoma and common adverse events were hyperglycemia [57%], hypertension [55%] and diarrhea [41%]; ALT elevations occurred in 26% of patients and were above 5 times ULN in 4%).
- Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018; 15: 273-91. [PubMed: 29508857](Thorough review of the role of PI3K in cellular pathways and the promise of specific inhibitors as therapy of metabolic and neoplastic diseases).
- Copanlisib (Aliqopa) for relapsed follicular lymphoma. Med Lett Drugs Ther 2018; 60 (1545): e74-e75. [PubMed: 29667951](Concise review of the mechanism of action, clinical efficacy, safety and costs of copanlisib shortly after its approval in the US for relapsed follicular lymphoma mentions adverse effects including hyperglycemia, leukopenia, hypertension and lung infections, but not ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Copanlisib for the Treatment of Malignant Lymphoma: Clinical Experience and Future Perspectives.[Target Oncol. 2021]Review Copanlisib for the Treatment of Malignant Lymphoma: Clinical Experience and Future Perspectives.Munoz J, Follows GA, Nastoupil LJ. Target Oncol. 2021 May; 16(3):295-308. Epub 2021 Mar 9.
- Review Copanlisib for the treatment of adults with relapsed follicular lymphoma.[Expert Rev Clin Pharmacol. 2020]Review Copanlisib for the treatment of adults with relapsed follicular lymphoma.Magagnoli M, Carlo-Stella C, Santoro A. Expert Rev Clin Pharmacol. 2020 Aug; 13(8):813-823. Epub 2020 Jul 2.
- Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma.[Ann Oncol. 2017]Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma.Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, Verhoef G, Linton K, Thieblemont C, Vitolo U, et al. Ann Oncol. 2017 Sep 1; 28(9):2169-2178.
- Review Spotlight on copanlisib and its potential in the treatment of relapsed/refractory follicular lymphoma: evidence to date.[Onco Targets Ther. 2018]Review Spotlight on copanlisib and its potential in the treatment of relapsed/refractory follicular lymphoma: evidence to date.Mensah FA, Blaize JP, Bryan LJ. Onco Targets Ther. 2018; 11:4817-4827. Epub 2018 Aug 13.
- Review Copanlisib: An Intravenous Phosphatidylinositol 3-Kinase (PI3K) Inhibitor for the Treatment of Relapsed Follicular Lymphoma.[Ann Pharmacother. 2019]Review Copanlisib: An Intravenous Phosphatidylinositol 3-Kinase (PI3K) Inhibitor for the Treatment of Relapsed Follicular Lymphoma.Eltantawy A, Vallejos X, Sebea E, Evans K. Ann Pharmacother. 2019 Sep; 53(9):954-958. Epub 2019 Feb 27.
- Copanlisib - LiverToxCopanlisib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...