NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Clomiphene is an oral agent used to treat infertility in women desiring pregnancy. Clomiphene has been linked to a low rate of transient serum aminotransferase elevations during therapy and to rare instances of clinically apparent liver injury, which can be severe and even fatal.
Background
Clomiphene (kloe' mi feen) is an orally available, nonsteroidal antiestrogen that stimulates ovulation and is used largely as therapy of infertility in women. Clomiphene interacts with estrogen receptors in many tissues, including the hypothalamus, pituitary, ovary, endometrium, vagina and cervix. The inhibition of estrogen effects by clomiphene leads to release of pituitary follicular stimulating hormone (FSH), which initiates growth and maturation of the ovarian follicle, follicular rupture and ovulation. In clinical trials, clomiphene resulted in pregnancies in approximately 30% of patients. Clomiphene was approved for use in the United States in 1967, and current indications are for treatment of ovulatory dysfunction in women desiring pregnancy. Clomiphene is available in tablets of 50 mg generically and under the brand name Clomid. The recommended dose is 50 mg daily for 5 days. Common side effects include headache, nausea, anorexia, diarrhea, rash and renal dysfunction. Uncommon, but potentially severe adverse events include hypersensitivity reactions, visual disturbance, abdominal pain, multiple pregnancies, and ovarian hyperstimulation syndrome.
Hepatotoxicity
There is little information on serum aminotransferase levels during clomiphene therapy which is typically given in low doses for a short time only. There have been a few reports of mild serum enzyme elevations in patients taking clomiphene, but no convincing instances of idiosyncratic, clinically apparent liver injury with its use.
Drugs used to treat infertility in women typically act by stimulation of the ovarian follicles which can lead to the ovarian hyperstimulation syndrome (OHSS), which can occasionally be accompanied by serum enzyme elevations and even jaundice. This syndrome typically arises within 4 to 14 days of ovarian stimulation with gonadotropins or clomiphene and is characterized by the onset of abdominal pain and distension with ascites and enlarged ovaries and ovarian cysts. There can be marked fluid shifts with hemoconcentration and rapid onset of severe ascites and pleural effusions. Liver tests are elevated in 25% to 40% of patients with OHSS, typically with mild-to-moderate increases in ALT and AST values, but minimal or no elevations in serum bilirubin and alkaline phosphatase levels. The liver test abnormalities resolve with resolution of the OHSS, usually within 2 to 3 weeks of onset. In severe instances, OHSS can be fatal, but death is usually due to dehydration, shock and septicemia rather than hepatic failure. In typical cases with abnormal liver enzymes, liver histology reveals nonspecific changes of sinusoidal dilatation, mild fat accumulation and focal inflammatory infiltrates with macrophages and lymphocytes. OHSS is less common with clomiphene than with human chorionic gonadotropin (hCG) induction of ovulation.
Likelihood score: C (probable cause of clinically apparent liver injury as a part of the ovarian hyperstimulation syndrome).
Mechanism of Injury
The mechanism of injury accounting for serum enzyme elevations during clomiphene therapy is not known. Clomiphene is metabolized in the liver and has a prolonged half-life. The liver test abnormalities found during OHSS may be due to fluid shifts, hypovolemia and ischemia.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation. Clomiphene has not been implicated in cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There does not appear to be cross reactivity in risk for hepatic injury between clomiphene and other drugs used to treat infertility.
Drug Class: Infertility Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Clomiphene – Generic, Clomid®
DRUG CLASS
Infertility Agents
Product labeling at DailyMed, National Library of Medicine, NIH
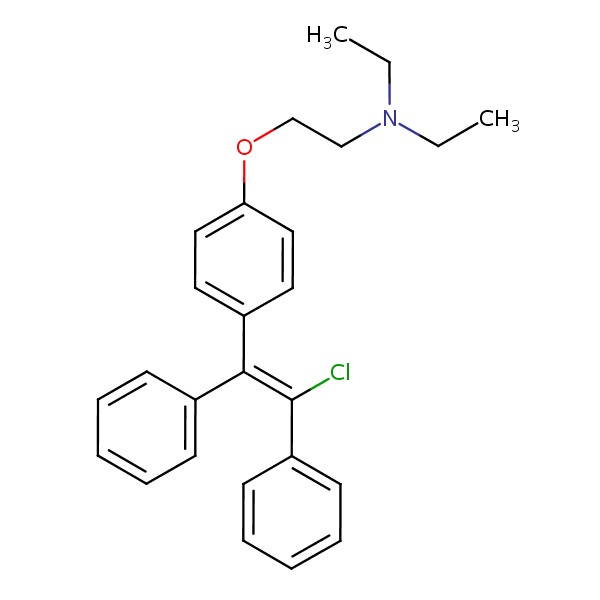
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Clomiphene | 911-45-5 | C26-H28-Cl-N-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 17 October 2017
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp 555-88.(Review of hepatotoxicity published in 1999; clomiphene is mentioned as having been linked unconvincingly to one case of hepatoblastoma [in a child born to a woman who received clomiphene] and one adenoma).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on hepatotoxicity; clomiphene not discussed).
- Schimmer BP, Parker KL. Contraception and pharmacotherapy of obstetrical and gynecological disorders. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1833-52.(Textbook of pharmacology and therapeutics).
- Bjork JT, Varma RR, Borkowf HI. Clomiphene citrate therapy in a patient with Laennec's cirrhosis. Gastroenterology 1977; 72: 1308-11. [PubMed: 858474](37 year old man with alcoholic cirrhosis who was abstinent from alcohol was treated with clomiphene with increase in FSH, LH and testosterone levels and improvement in impotence but not gynecomastia; mildly abnormal liver tests did not change during treatment).
- Lunenfeld B, Blankstein J, Kotev-Emeth S, Kokia E, Geier A. Drugs used in ovulation induction. Safety of patient and offspring. Hum Reprod 1986; 1: 435-9. [PubMed: 3106402](Review of safety of clomiphene and human chorionic gonadotropin used as therapy of infertility in women to induce ovulation; no mention of ALT elevations or hepatotoxicity).
- Younis JS, Zeevi D, Rabinowitz R, Laufer N, Schenker JG. Transient liver function tests abnormalities in ovarian hyperstimulation syndrome. Fertil Steril 1988; 50: 176-8. [PubMed: 3384112](2 women, ages 24 and 28 years, developed abdominal pain and distension, nausea and dyspnea 7 and 16 days after aspiration of oocytes and therapy with human chorionic gonadotropin [bilirubin 0.7 and 0.8 mg/dL, ALT 103 and 124 U/L, Alk P 94 and 115 U/L, albumin 2.0 and 3.3 g/dL], resolving with resolution of the ascites and ovarian enlargement within 3-4 weeks).
- Sueldo CE, Price HM, Bachenberg K, Steinleitner A, Gitlin N, Swanson J. Liver dysfunction in ovarian hyperstimulation syndrome. A case report. J Reprod Med 1988; 33: 387-90. [PubMed: 3130483](29 year old woman with infertility developed ascites and OHSS within a week of stopping a course of human menopausal gonadotropin [bilirubin 2.8 mg/dL, ALT 207 rising to 880 U/L, Alk P 111 U/L], liver biopsy was largely normal except for intramitochondrial paracrystaliine inclusions).
- Ryley NG, Forman R, Barlow D, Fleming KA, Trowell JM. Liver abnormality in ovarian hyperstimulation syndrome. Hum Reprod 1990; 5: 938-43. [PubMed: 1982004](32 year old woman with infertility treated with human gonadotropin and embryo transfer developed nausea, abdominal distension, marked ascites and hypovolemic shock [peak bilirubin 1.5 mg/dL, AST 255 U/L, Alk P 1173 U/L], biopsy showing fatty change and focal infiltrates, resolving after "evacuation of products").
- Balasch J, Carmona F, Llach J, Arroyo V, Jové I, Vanrell JA. Acute prerenal failure and liver dysfunction in a patient with severe ovarian hyperstimulation syndrome. Hum Reprod 1990; 5: 348-51. [PubMed: 2112558](27 year old woman with infertility developed abdominal pain, distension, ascites, nausea and dyspnea 5 days after follicular aspiration and treatment with hCG [bilirubin rising to 4.4 m/dL, ALT 49 U/L, Alk P 627 U/L], resolving within 2-3 weeks).
- Marsepoil T, Cordesse A, Varguy P, Dauptain G, Levesque P. [Hepatic involvement in the course of ovarian hyperstimulation syndrome]. Rev Fr Gynecol Obstet 1992; 87: 148-9. French. [PubMed: 1579800](22 year old woman with infertility developed OHSS shortly after receiving clomiphene and hCG with ascites and pleural effusions [ALT rising to 40 times ULN, bilirubin and Alk P normal], biopsy showing mild portal inflammation and sinusoidal dilatation without hepatocyte necrosis, resolving within 2 months).
- Senturk H, Erdinç, Tasyurekli M, Mert A, Arvas M, Sen C. Case report: liver function abnormalities in a severe case of hyperreactio luteinalis. J Gastroenterol Hepatol 1996; 11: 617-20. [PubMed: 8840234](A 12 week pregnant 19 year old developed multicystic ovarian masses and ascites with rises in liver tests [peak bilirubin 2.0 mg/dL, ALT 214 U/L], resolving within 2-3 weeks).
- Wakim AN, Fox SD. Elevated liver function tests in a case of moderate ovarian hyperstimulation syndrome. Hum Reprod 1996; 11: 588-9. [PubMed: 8671272](28 year old woman with infertility was treated with clomiphene and hCG for ovarian stimulation developed abdominal pain and ascites [ALT 183 U/L, bilirubin and Alk P normal], resolving within 2 weeks of onset at time of resolution of ascites).
- Nawroth F, Heinrich J, Bruns U, Wood WG. Severe ovarian hyperstimulation syndrome (OHSS) and icterus. Hum Reprod 1996; 11: 2441-2. [PubMed: 8981129](33 year old woman with infertility treated with clomiphene and HCG developed nausea and distended abdomen with ascites and enlarged ovaries [bilirubin rising to 5.2 mg/dL, ALT 184 U/L, Alk P normal], resolving within 2-3 weeks).
- Rizk B, Aboulghar M, Smitz J, Ron-El R. The role of vascular endothelial growth factor and interleukins in the pathogenesis of severe ovarian hyperstimulation syndrome. Hum Reprod Update 1997; 3: 255-66. [PubMed: 9322101](Review of OHSS including clinical presentation with massive cystic enlargement of ovaries and fluid shift with ascites, pleural effusions and hypovolemia caused by cytokines and growth factors [VEGF] induced by ovarian stimulation by gonadotropin therapy).
- Shimono J, Tsuji H, Azuma K, Hashiguchi M, Fujishima M. A rare case of hepatic injury associated with ovarian hyperstimulation syndrome. Am J Gastroenterol 1998; 93 (1): 123-4. [PubMed: 9448194](26 year old woman treated with menopausal gonadotropin and clomiphene to prevent spontaneous abortion developed massive ascites and multiple ovarian cysts [bilirubin 1.3 mg/dL, ALT 194 U/L], with subsequent resolution, but spontaneous abortion a few weeks later).
- Midgley DY, Khalaf Y, Braude PR, Nelson-Piercy C. Recurrent cholestasis following ovarian hyperstimulation syndrome: case report. Hum Reprod 1999; 14: 2249-51. [PubMed: 10469689](34 year old woman with infertility developed OHSS 4 weeks after successful embryo transfer with ascites and enlarged ovaries [ALT 65 U/L] and then at 9 weeks developed pruritus and cholestasis of pregnancy [ALT 215 U/L, elevated bile acids], and then developed preeclampsia and cholestasis again [ALT ~350 U/L], resolving with delivery of normal twins).
- Fábregues F, Balasch J, Ginès P, Manau D, Jiménez W, Arroyo V, Creus M, et al. Ascites and liver test abnormalities during severe ovarian hyperstimulation syndrome. Am J Gastroenterol 1999; 94: 994-9. [PubMed: 10201472](Among 50 women with OHSS admitted over a 7 year period to a single referral center, all developed ascites between 4 and 11 days after ovulation induction with gonadotropins, 15 had abnormal liver tests [bilirubin normal, ALT 51-277 U/L, Alk P 83-411 U/L], resolving within 1-3 weeks along with ascites).
- Chen CD, Wu MY, Chen HF, Chen SU, Ho HN, Yang YS. Relationships of serum pro-inflammatory cytokines and vascular endothelial growth factor with liver dysfunction in severe ovarian hyperstimulation syndrome. Hum Reprod 2000; 15: 66-71. [PubMed: 10611190](Among 29 women with OHSS after in vitro fertilization, 15 had abnormal liver tests [bilirubin 1.2-2.6 mg/dL, ALT 35-144 U/L, Alk P 64-238 U/L]).
- Elter K, Scoccia B, Nelson LR. Hepatic dysfunction associated with moderate ovarian hyperstimulation syndrome. A case report. J Reprod Med 2001; 46: 765-8. [PubMed: 11547654](33 year old woman with infertility developed abdominal distension, ascites and OHSS 11 days after embryo transfer, with daily injections of leuprolide and several injections of FSH, hCG and progesterone [ALT 265 U/L, bilirubin and Alk P normal], resolving in 1-2 weeks).
- Davis AJ, Pandher GK, Masson GM, Sheron N. A severe case of ovarian hyperstimulation syndrome with liver dysfunction and malnutrition. Eur J Gastroenterol Hepatol 2002; 14: 779-82. [PubMed: 12169989](28 year old woman with infertility developed abdominal pain and distension 1 day after embryo transfer [bilirubin 0.6 mg/dL, ALT 462 U/L, Alk P 706 U/L, prothrombin time 19 sec], resolving over the ensuing 3 weeks).
- Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS). Hum Reprod Update 2003; 9: 77-96. [PubMed: 12638783](Review of OHSS mentions that liver abnormalities occur in 26-40% of cases, typically with elevations in ALT, but normal bilirubin and Alk P values, resolving in all cases with resolution of the syndrome).
- Obrzut B, Kuczyński W, Grygoruk C, Putowski L, Kluz S, Skret A. Liver dysfunction in severe ovarian hyperstimulation syndrome. Gynecol Endocrinol 2005; 21: 45-9. [PubMed: 16048801](32 year old woman with infertility developed severe OHSS with marked ascites, dyspnea and hypovolemia after therapy with clomiphene and human chorionic gonadotropin [bilirubin 5 mg/dL, ALT 3372 U/L, Alk P 310 U/L], with full recovery over the ensuing month).
- Binder H, Dittrich R, Einhaus F, Krieg J, Müller A, Strauss R, Beckmann MW, Cupisti S. Update on ovarian hyperstimulation syndrome: Part 1--Incidence and pathogenesis. Int J Fertil Womens Med 2007; 52: 11-26. [PubMed: 17987884](Review of OHSS, which arises in 0.2-1% of stimulation cycles in assisted reproduction and may be mediated by VEGF levels of which correlate with hCG levels; liver enzyme elevations, predominantly ALT and AST, arise in 25-40% of cases possibly due to the increased capillary permeability of OHSS).
- Mitchell C, Gottlieb L. Transaminitis after treatment with clomiphene citrate: a case report. J Reprod Med 2007; 52 437-8. [PubMed: 17583249](30 year old woman developed severe abdominal pain a few days after each of two courses of clomiphene [bilirubin 1.3 mg/dL, ALT 338 U/L, Alk P 87 U/L], ultrasound showing fatty liver without ascites, resolving within 2 months of onset).
- Drugs for ovulation induction. Med Lett Drugs Ther 2011; 53 (1376): 86-8. [PubMed: 22033212](Concise review of the efficacy and safety of drugs used for ovarian induction mentions that common side effects of clomiphene are hot flushes, gastrointestinal upset, headache, breast discomfort and uterine bleeding and that clomiphene, particularly in combination with FSH or LH, can cause OHSS which can be severe and even fatal; no mention of ALT elevations or hepatotoxicity).
- Figueiredo JB, Nastri CO, Vieira AD, Martins WP. Clomiphene combined with gonadotropins and GnRH antagonist versus conventional controlled ovarian hyperstimulation without clomiphene in women undergoing assisted reproductive techniques: systematic review and meta-analysis. Arch Gynecol Obstet 2013; 287: 779-90. [PubMed: 23250342](Review of 7 studies comparing the safety of regimens to induce ovulation with or without clomiphene mentions that OHSS is less common in regimens that include clomiphene [0.5% vs 4%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to clomiphene or other agents used for infertility).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Deferasirox.[LiverTox: Clinical and Researc...]Review Deferasirox.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Sorafenib.[LiverTox: Clinical and Researc...]Review Sorafenib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome.[Cochrane Database Syst Rev. 2016]Review Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome.Brown J, Farquhar C. Cochrane Database Syst Rev. 2016 Dec 15; 12(12):CD002249. Epub 2016 Dec 15.
- Review Deferiprone.[LiverTox: Clinical and Researc...]Review Deferiprone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- [Fertility disorders in 49 hyperandrogenic women desiring pregnancy. Treatment results aimed at obtaining a pregnancy in 40 hyperandrogenic infertile women and in 9 hyperandrogenic women desiring pregnancy].[Rev Fr Gynecol Obstet. 1994][Fertility disorders in 49 hyperandrogenic women desiring pregnancy. Treatment results aimed at obtaining a pregnancy in 40 hyperandrogenic infertile women and in 9 hyperandrogenic women desiring pregnancy].Cordray JP, Merceron RE, Siboulet B, Guillerd X, Nys P, Reboul P, Rainaut M. Rev Fr Gynecol Obstet. 1994 May; 89(5):267-74.
- Clomiphene - LiverToxClomiphene - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...