NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Buprenorphine is a sublingually and transdermally available, semisynthetic opioid analgesic, which is used as an analgesic and for management of opioid dependence. Therapy with buprenorphine is associated with mild and transient serum enzyme elevations, and with moderate-to-severe clinically apparent liver injury when abused by intravenous administration or the sublingual formations.
Background
Buprenorphine (bue" pre nor' feen) is a semisynthetic opioid that is 25 to 50 times more potent than morphine and has been used as an analgesic as well as therapy of opioid addiction. Buprenorphine is a partial µ-opioid receptor agonist and a κ-receptor antagonist accounting for its benefit for opioid deterrence. Buprenorphine competes with morphine and heroin for the µ receptor, but is only a partial agonist and has a “ceiling” effect. Buprenorphine was approved for treatment of opioid addiction in 2004 and is a schedule III controlled substance. Current indications include treatment of moderate-to-severe pain (parenterally in low doses <1 mg) and opiate addiction (in various formulations in higher doses 2 to 16 mg daily). Buprenorphine is available as 2 and 8 mg tablets for sublingual administration generically and under the brand name Subutex, and in 1 mL ampules of 0.3 mg/mL for intravenous (iv) or intramuscular (im) injection generically and under the brand name Buprenex. More recently, buccal film formulations, transdermal patches (Butrans and generics), once monthly prolonged release injections (Sublocade) and once every 6 months implants injected subcutaneously (Probuphine) with buprenorphine have become available as therapy for opioid dependence. Buprenorphine is also available in fixed combination with naloxone (tablets and buccal films: 2 mg/0.5 mg and 8 mg/2 mg) for sublingual administration generically and under the brand name Suboxone. Naloxone is not absorbed orally, but provides full opioid antagonism if the combination is administer intravenously, as might occur with intentional abuse. Oral naloxone may also help to alleviate the gastrointestinal side effects of opioids such as constipation, nausea, vomiting and abdominal bloating. Finally, parenteral forms of buprenorphine are used for moderate-to-severe pain and administered iv or im, the typical dose being 0.3 mg every 6 hours as needed. Common side effects of buprenorphine include headache, dizziness, fatigue, sedation, dry mouth, urinary retention, diaphoresis, orthostatic hypotension, biliary spasm and withdrawal symptoms. Rare but potentially severe adverse reactions include life threatening respiratory and CNS depression, hepatotoxicity, adrenal insufficiency, interactions with other sedative medications, unintentional pediatric exposure and neonatal opioid withdrawal syndrome. Buprenorphine should be prescribed only by a health care provider with training in opioid dependency and managing the dosing and side effects of buprenorphine and naloxone.
Hepatotoxicity
Buprenorphine therapy has been associated with a low rate of serum enzyme elevations during treatment, although the populations studied (opioid dependent) often have coexisting chronic liver diseases which complicate such assessments. Nevertheless, rates of ALT elevations during treatment with buprenorphine (with or without naloxone) have been minimally or no greater than with comparator arms (methadone).
In addition, there have been several reports and case series of acute, clinically apparent liver injury arising within 2 to 20 weeks of starting buprenorphine, usually (but not invariably) following misuse and intravenous administration of sublingual tablets. However, some cases occurred in patients who denied intravenous use and were on conventional sublingual doses. In most cases, the pattern of serum enzyme elevations was hepatocellular and the presentation resembled acute toxic hepatic necrosis. Immunoallergic features (fever, rash and eosinophilia) were not present, nor were autoantibodies detected. Almost all patients with this injury had concurrent chronic hepatitis C, and several appeared to resolve the chronic infection with the acute liver injury (Case 1). Strikingly, most patients were able to continue buprenorphine without recurrence, some of whom admitted to continued intravenous abuse.
Likelihood score: B[HD] (likely rare cause of clinically apparent liver injury usually associated with overdose or misuse).
Mechanism of Injury
Buprenorphine undergoes extensive first pass hepatic extraction and is metabolized primarily by the cytochrome P450 system (CYP 3A4). The low doses used and rapid metabolism may account for its relative lack of hepatotoxicity when used in conventional doses. The appearance of an acute hepatic necrosis after intravenous injection of the sublingual tablets is likely due to direct toxicity of these high parenteral doses. The occurrence of this syndrome largely in patients with concurrent hepatitis C remains unexplained. Clearance of hepatitis C during this toxic injury raises the issue of whether the acute injury is actually due to an exacerbation of hepatitis C or whether the severe acute toxic injury can induce viral clearance.
Outcome and Management
The severity of liver injury attributed to buprenorphine has ranged from mild-to-severe hepatitis, and fatal instances have been reported. Interestingly, most patients subsequently tolerated restarting buprenorphine at doses used prior to the toxic event, and some patients continued to misuse it by intravenous injection, but did not have recurrence of the liver injury.
Drug Class: Substance Abuse Treatment Agents; Opioids
CASE REPORT
Case 1. Acute liver injury attributed to buprenorphine use in a patient with chronic hepatitis C.(1)
A 33 year old male injection drug user with chronic hepatitis C (HCV genotype 1a) developed jaundice, abdominal pain and fever shortly after injecting 3 to 5 crushed and dissolved tablets of buprenorphine (8 mg, meant for sublingual administration) intravenously. He was known to have chronic hepatitis C, but had never been treated for the infection which was considered mild. He had no other history of liver disease but consumed alcohol regularly. He took no other medications. On admission, laboratory tests showed a serum bilirubin of 29.6 mg/dL with marked elevations in serum aminotransferase levels (ALT 4132 U/L, AST 3947 U/L) and mild increases in alkaline phosphatase (347 U/L) and GGT (127 U/L; normal <50) (Table). The prothrombin index was 43% of control (INR 1.7). A transjugular liver biopsy showed panlobular necrosis, diffuse lobular inflammation, and marked cholestasis indicative of acute hepatic injury. In addition, there was moderate portal fibrosis and portal inflammation suggestive of an underlying chronic hepatitis C. Tests for hepatitis A and B and HIV were negative. Anti-HCV was present, but serum HCV RNA was present at very low levels (15 IU/mL). Jaundice resolved within a week and serum enzymes fell rapidly. Sublingual buprenorphine was restarted without a change in the pattern of recovery. In follow up 3 months later, serum ALT levels were normal and HCV RNA was undetectable. When seen almost a year later, he admitted to continuing to self-inject buprenorphine, yet serum ALT levels were still normal and HCV RNA was undetectable.
Key Points
| Medication: | Buprenorphine (24 mg intravenously) |
|---|---|
| Pattern: | Hepatocellular (R=31) |
| Severity: | 4+ (jaundice and prolongation of INR) |
| Latency: | Short, specific time not provided |
| Recovery: | Rapid, within 1-2 months |
| Other medications: | None |
Laboratory Values
| Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|
| 0 | 4132 | 347 | 29.6 | Admission |
| 2 days | 2546 | 288 | 26.9 | Prothrombin time 18 sec |
| 2 weeks | 132 | 80 | 2.2 | Buprenorphine restarted |
| 4 months | Normal | HCV RNA negative | ||
| 10 months | 11 | HCV RNA negative | ||
| Normal | <40 | <104 | <1.2 |
Comment
The clinical presentation of acute hepatic necrosis shortly after intravenous abuse of buprenorphine has been reported in several case reports and single center series of as many as 7 cases. Intriguingly, virtually all patients reported with this syndrome had a preexisting chronic hepatitis C, although many were HCV RNA negative at the time of the acute illness. The current case documents the loss of HCV RNA during the acute hepatic injury attributed to buprenorphine. Two explanations are possible. First, that the acute toxic hepatic injury caused an interruption in the replication of HCV or triggered host immune responses (the innate immune system and interferon production), resulting in clearance of hepatitis C. A second explanation is that the acute illness was due to an exacerbation of the chronic HCV infection, which led to an acute hepatitis C-like clearance of virus. The role of hepatitis C in this reaction is perhaps demonstrated best by the absence of recurrence of liver injury when buprenorphine was restarted and even when abused intravenously in high doses. The combination of buprenorphine with naloxone (a full µ opioid receptor antagonist) was developed to discourage such intravenous abuse.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Buprenorphine – Generic, Buprenex®, Subutex® (SL Tablets)
Buprenorphine/Naloxone – Generic, Suboxone®
DRUG CLASS
Substance Abuse Treatment Agents
Product labeling at DailyMed, National Library of Medicine, NIH
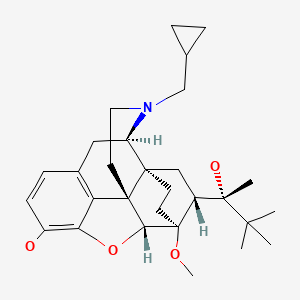
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Buprenorphine | 52485-79-7 | C29-H41-N-O4 |
|
CITED REFERENCES
- 1.
- Peyrière H, Tatem L, Bories C, Pageaux GP, Blayac JP, Larrey D. Hepatitis after intravenous injection of sublingual buprenorphine in acute hepatitis C carriers: report of two cases of disappearance of viral replication after acute hepatitis. Ann Pharmacother. 2009;43:973–7. [Modified from Case 1.] [PubMed: 19383935]
ANNOTATED BIBLIOGRAPHY
References updated: 24 November 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 before the availability of buprenorphine).
- Larrey D, Ripault M-P. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of hepatotoxicity of drugs of abuse mentions that hepatotoxicity from buprenorphine is rare when used in recommended sublingual doses, but that severe reactions occur when used by the intravenous route, possibly as a result of mitochondrial injury).
- Yaksh T, Wallace M. Opioids, analgesia, and pain management. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 355-386.(Textbook of pharmacology and therapeutics).
- Hirschauer C, Guillemot F, Héud M, Mathieu-Chandelier C, Cortot A, Paris JC. Gastroenterol Clin Biol. 1989;13:636. [Is buprenorphine hepatotoxic?] French. [PubMed: 2753313](36 year old man developed abnormal liver tests 3 months after starting buprenorphine for chronic pain [bilirubin 0.6 mg/dL, ALT 100 U/L, GGT 899 U/L], resolving rapidly, but rising again on rechallenge [ALT 52 U/L]; biopsy showed steatosis and inflammation, and the patient admitted to alcohol use [50 g/day]).
- Houdret N, Asnar V, Szostak-Talbodec N, Leteurtre E, Humbert L, Lecomte-Houcke M, Lhermitte M, Paris JC. Acta Clin Belg. 1999;53 Suppl 1:29–31. [Hepatonephritis and massive ingestion of buprenorphine] French. [PubMed: 10216978](33 year old man developed hepatic and renal failure 1-2 days after taking an overdose of buprenorphine [112 mg] [bilirubin 5.5 mg/dL, ALT 11,080 U/L, Alk P 334 U/L, prothrombin index 20%, creatinine 8.0 mg/dL], with subsequent rapid and complete recovery).
- Berson A, Gervais A, Cazals D, Boyer N, Durand F, Bernuau J, Marcellin P, et al. Hepatitis after intravenous buprenorphine misuse in heroin addicts. J Hepatol. 2001;34:346–50. [PubMed: 11281569](Five cases of acute hepatitis-like injury in male heroin users, ages 25 to 34 years, arising after 2-8 weeks of buprenorphine therapy, 4 admitted to iv injection [bilirubin 0.8-11 mg/dL, ALT 520-6595 U/L, Alk P 126-306 U/L], one with hepatic failure, rapidly resolving, but all 5 had chronic hepatitis C, one had concurrent hepatitis B and one had HIV infection; several were continued on sublingual buprenorphine without recurrence).
- Wisniewski B, Perlemuter G, Buffet C. Gastroenterol Clin Biol. 2001;25:328–9. [Acute hepatitis following intravenous buprenorphine injection as a substitute drug in a drug-addict] French. [PubMed: 11395686](25 year old with chronic hepatitis C and heroin use developed jaundice 72 hours after injecting buprenorphine intravenously [bilirubin 4.3 mg/dL, ALT 31 times ULN, Alk P 2.5 times ULN, HCV RNA positive], biopsy showing acute hepatitis and cholestasis; rapid recovery).
- Petitjean S, Stohler R, Deglon JJ, Livoti S, Waldvogel D, Uehlinger C, Ladewig D. Double-blind randomized trial of buprenorphine and methadone in opiate dependence. Drug Alcohol Depend. 2001;62:97–104. [PubMed: 11173173](Controlled trial comparing methadone to buprenorphine in 58 patients with opioid dependence found poorer retention rate with buprenorphine; no hepatotoxicity reported).
- Vidal-Trecan G, Varescon I, Nabet N, Boissonnas A. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alcohol Depend. 2003;69:175–81. [PubMed: 12609698](Survey of 404 subjects on maintenance buprenorphine for opiate abuse found that 46% admitted to illicit intravenous self-administration).
- Mattick RP, Ali R, White JM, O'Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98:441–52. [PubMed: 12653814](Controlled trial in 405 patients with opioid dependence found similar rates of adverse events with buprenorphine and methadone; one patient had hepatitis C, but no other liver related adverse events reported).
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, et al. Buprenorphine/Naloxone Collaborative Study Group Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–58. [PubMed: 12954743](Controlled trial of buprenorphine/naloxone vs buprenorphine alone vs placebo for 4 weeks in 326 opiate addicted patients; ALT elevations occurred in 10 patients, but 8 had concurrent hepatitis B or C).
- Hervé S, Riachi G, Noblet C, Guillement N, Tanasescu S, Goria O, Thuillez C, et al. Acute hepatitis due to buprenorphine administration. Eur J Gastroenterol Hepatol. 2004;16:1033–7. [PubMed: 15371928](7 cases of hepatotoxicity attributed to buprenorphine, all heroin addicts, 6 men and 1 woman, ages 24 to 38 years on buprenorphine [2-12 mg/day] for 4-20 weeks, sublingual in 6 and by injection in one, all had anti-HCV and 2 HCV RNA, 2 HBsAg [ALT 9 to 68 times ULN], 5 with jaundice [bilirubin levels not given]; all recovered rapidly despite continuing buprenorphine [at reduced doses in 3]).
- Zuin M, Giorgini A, Selmi C, Battezzati PM, Cocchi CA, Crosignani A, Benetti A, et al. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Dig Liver Dis. 2009;41:e8–e10. [PubMed: 18294936](33 year old man with chronic hepatitis C and heroin use developed jaundice 3 weeks after starting sublingual buprenorphine [20 mg daily] [bilirubin 8.4 mg/dL, ALT 300 times ULN, INR 3.1, creatinine 4.6, HCV RNA positivity], but rapid recovery and ALT levels normal and HCV RNA negative in follow-up).
- Peyrière H, Tatem L, Bories C, Pageaux GP, Blayac JP, Larrey D. Hepatitis after intravenous injection of sublingual buprenorphine in acute hepatitis C carriers: report of two cases of disappearance of viral replication after acute hepatitis. Ann Pharmacother. 2009;43:973–7. [PubMed: 19383935](Two male injection drug users, 33 and 50 years old, with chronic hepatitis C presented with jaundice after injecting buprenorphine [bilirubin 29.6 and 2.1 mg/dL, ALT 4132 and 866 U/L, Alk P 347 and 1028 U/L], resolving rapidly and associated with loss of HCV RNA: Case 1).
- Orman JS, Keating GM. Buprenorphine/naloxone: a review of its use in the treatment of opioid dependence. Drugs. 2009;69:577–607. [PubMed: 19368419](Review of the pharmacology, safety and efficacy of the combination of buprenorphine and naloxone as therapy of opioid dependence; no report of clinically apparent liver injury in preregistration clinical trials).
- Pinto H, Maskrey V, Swift L, Rumball D, Wagle A, Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39:340–52. [PubMed: 20817384](Patient preference controlled trial comparing buprenorphine and methadone for up to 6 months in 361 opiate dependent subjects; no discussion of hepatotoxicity or ALT elevations).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to buprenorphine or other agents used to treat substance abuse).
- Vergara-Rodriguez P, Tozzi MJ, Botsko M, Nandi V, Altice F, Egan JE, O'Connor PG, et al. BHIVES Collaborative. Hepatic safety and lack of antiretroviral interactions with buprenorphine/naloxone in HIV-infected opioid-dependent patients. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S62–7. [PubMed: 21317596](Among 141 HIV infected subjects treated with buprenorphine/naloxone for 6 months, median serum ALT and AST values did not change and no patient developed clinically apparent liver injury, 3 had transient ALT elevations during treatment [383, 123 and 218 U/L], but all resolved either spontaneously or with drug discontinuation).
- Dasgupta N, Bailey EJ, Cicero T, Inciardi J, Parrino M, Rosenblum A, Dart RC. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010;11:1078–91. [PubMed: 20545875](Between 2003 and 2007 there were increases in the rates of abuse and diversion of both methadone and buprenorphine in the US, the rates being higher for methadone).
- Upadhyay A, Xueming Y. Buprenorphine-induced elevated liver enzymes in an adolescent patient. J Child Adolesc Psychopharmacol. 2010;20:545–6. [PubMed: 21186976](17 year old girl developed abdominal pain and enzyme elevations 9 days after starting buprenorphine with naloxone [bilirubin normal, ALT 452 U/L, anti-HCV negative], resolving rapidly on stopping; also taking quetiapine and tizanidine).
- Bogenschutz MP, Abbott PJ, Kushner R, Tonigan JS, Woody GE. Effects of buprenorphine and hepatitis C on liver enzymes in adolescents and young adults. J Addict Med. 2010;4:211–6. [PMC free article: PMC3002235] [PubMed: 21170166](Among 152 patients with opioid dependence treated with 2 vs 12 weeks of buprenorphine with naloxone, ALT elevations occurred in 13% vs 23% of patients, but were mostly attributed to underlying HCV infection).
- Kraus ML, Alford DP, Kotz MM, Levounis P, Mandell TW, Meyer M, Salsitz EA, et al. Statement of the American Society of Addiction Medicine Consensus Panel on the Use of Buprenorphine in Office-Based Treatment of Opioid Addiction. J Addict Med. 2011;5:254–63. [PubMed: 22042215](Guidelines and recommendations for use of buprenorphine in office based practice; no discussion of hepatotoxicity).
- Steiner DJ, Sitar S, Wen W, Sawyerr G, Munera C, Ripa SR, Landau C. Efficacy and safety of the seven-day buprenorphine transdermal system in opioid-naïve patients with moderate to severe chronic low back pain: an enriched, randomized, double-blind, placebo-controlled study. J Pain Symptom Manage. 2011;42:903–17. [PubMed: 21945130](541 patients with severe low back pain were randomized to receive transdermal buprenorphine or placebo for 12 weeks; overall, clinical laboratory test results did not change on buprenorphine treatment).
- Amass L, Pukeleviciene V, Subata E, Almeida AR, Pieri MC, D'Egidio P, Stankova Z, et al. A prospective, randomized, multicenter acceptability and safety study of direct buprenorphine/naloxone induction in heroin-dependent individuals. Addiction. 2012;107:142–51. [PubMed: 21749526](Among 187 injection drug users treated for 4 weeks with buprenorphine with naloxone, common side effects included insomnia, sweating, nausea and anxiety, but all serious adverse events were considered unrelated; no mention of ALT elevations).
- Ray R, Vaswani M, Deb KS, Sethi H, Chopra A, Kishore N, Goyal R. Post marketing surveillance of sublingual buprenorphine naloxone combination tablets. Indian J Physiol Pharmacol. 2012;56:359–66. [PubMed: 23781656](Among 158 patients with opioid dependence treated with buprenorphine with naloxone, liver enzyme elevations were reported in 52% of patients, but none was associated with severe hepatitis).
- Saxon AJ, Ling W, Hillhouse M, Thomas C, Hasson A, Ang A, Doraimani G, et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128:71–6. [PMC free article: PMC3543467] [PubMed: 22921476](Among 731 patients with opioid dependency treated with either buprenorphine with naloxone or methadone for at least 24 weeks, 12.6% vs 17.9% developed ALT elevations [>2 times ULN], which were "extreme" in only 2.1% vs 3.6% [bilirubin 0.7-3.7 mg/dL, ALT 418-6280 U/L], usually attributable to underlying HBV or HCV infection, but specific details were not provided).
- Soyka M, Backmund M, Schmidt P, Apelt S. Buprenorphine-naloxone treatment in opioid dependence and risk of liver enzyme elevation: results from a 12-month observational study. Am J Addict. 2014;23:563–9. [PubMed: 25251050](Among 188 opioid dependent patients treated with buprenorphine/naloxone for 12 months, 1-2% had liver enzyme elevations that were “mostly discrete” [all less than 100 U/L], transient and without jaundice or symptoms).
- Lucas GM, Young A, Donnell D, Richardson P, Aramrattana A, Shao Y, Ruan Y, et al. HPTN 058 study group. Hepatotoxicity in a 52-week randomized trial of short-term versus long-term treatment with buprenorphine/naloxone in HIV-negative injection opioid users in China and Thailand. Drug Alcohol Depend. 2014;142:139–45. [PMC free article: PMC4127183] [PubMed: 24999060](Among 1036 patients with opioid use disorder treated with buprenorphine/naloxone in short [18 day] or long [52 week] courses, serum ALT elevations above 3 times ULN arose in 76 patients [7%], most instances occurring with either acute or chronic HCV infection and rates of ALT elevations were similar with either short and long term use, as well as after discontinuation of the medication).
- Nasser AF, Heidbreder C, Liu Y, Fudala PJ. Pharmacokinetics of sublingual buprenorphine and naloxone in subjects with mild to severe hepatic impairment (Child-Pugh Classes A, B, and C), in hepatitis C virus-seropositive subjects, and in healthy volunteers. Clin Pharmacokinet. 2015;54:837–49. [PubMed: 25603822](Pharmacokinetic study of sublingual buprenorphine and naloxone in 33 patients with variable degrees of hepatic dysfunction identified higher plasma concentrations of both agents in patients with moderate or severe dysfunction (Child’s Pugh B and C], but not in those with mild dysfunction or chronic HCV infection without cirrhosis; side effects included dizziness, nausea, fatigue and pruritus but there were “no post-dose findings of clinical relevance in clinical laboratory” tests ).
- French J, Mujumdar A, Angus P, Gow P. Fulminant hepatic failure after intravenous injection of sublingual buprenorphine in a patient with hepatitis C. Clin Case Rep. 2015;3:705–6. [PMC free article: PMC4551330] [PubMed: 26331017](20 year old indigenous Australian male with known chronic hepatitis C and injection drug abuse developed severe hepatitis with jaundice arising a day after injecting buprenorphine/naloxone meant for sublingual use [bilirubin 13.8 mg rising to 26.4 mg/dL, ALT 8768 U/L, INR 9, lactate 5.6 ], with rapid recovery with medical management, no follow up of HCV status after recovery).
- Ciftci Demirci A, Gunes H, Adaletli H, Bulanik E, Erdogan A. Liver enzyme levels in adolescent patients treated with buprenorphine and additional psychotropic agents. Am J Drug Alcohol Abuse. 2015;41:107–13. [PubMed: 25490611](Among 59 heroin-dependent adolescents started on buprenorphine/naloxone, mean serum ALT and AST levels rose by week 2 but returned to baseline levels by week 8 of therapy; one patient developed acute hepatitis C during treatment).
- Tetrault JM, Tate JP, Edelman EJ, Gordon AJ, Lo Re V 3rd, Lim JK, Rimland D, et al. Hepatic safety of buprenorphine in HIV-infected and uninfected patients with opioid use disorder: the role of HCV-infection. J Subst Abuse Treat. 2016;68:62–7. [PMC free article: PMC4976086] [PubMed: 27431048](Among 666 patients [mostly men] identified in a Veterans Administration electronic medical database who were started on buprenorphine and were monitored, 14 developed “drug-induced liver injury”, all of whom had received a potentially hepatotoxic medication [93%] or had preexisting HCV infection [7%], and there was no overall “substantial” change in serum ALT, AST or bilirubin levels).
- Aldemir E, Coskunol H, Kilic M, Sert I. Treatment of opioid dependence with buprenorphine/naloxone after liver transplantation: Report of two cases. Transplant Proc. 2016;48:2769–72. [PubMed: 27788815](Two patients with liver transplants for chronic hepatitis C and opioid use disorder were treated successfully with buprenorphine/naloxone with no significant side effects or worsening of minimal ALT and AST elevations due to recurrent hepatitis C ).
- Buprenorphine implants (Probuphine) for opioid dependence. Med Lett Drugs Ther. 2016;58(1499):94–5. [PubMed: 27403784](Concise summary of the mechanism of action, efficacy, safety and costs of a subdermally administered implant that provides plasma levels of buprenorphine for 6 months and appears to be similar in efficacy to transdermal or buccal administration, but has added adverse effects such as injury from the insertion and removal of the implant; mentions that hepatotoxicity has occurred with buprenorphine therapy).
- Buprenorphine buccal film (Belbuca) for chronic pain. Med Lett Drugs Ther. 2016;58(1492):47–8. [PubMed: 27049508](Concise summary of the mechanism of action, efficacy, safety and costs of a new formulation of buprenorphine provided as a buccal film).
- Pergolizzi JV, Raffa RB, Marcum Z, Colucci S, Ripa SR. Safety of buprenorphine transdermal system in the management of pain in older adults. Postgrad Med. 2017;129:92–101. [PubMed: 27929709](Review of the literature on the safety and efficacy of buprenorphine in elderly patients mentions that liver abnormalities are rare [<1%] and ALT values above 3 times ULN occur in only 0.2% of both elderly and younger adults).
- Concheiro M, Chesser R, Pardi J, Cooper G. Postmortem toxicology of new synthetic opioids. Front Pharmacol. 2018;9:1210. [PMC free article: PMC6212520] [PubMed: 30416445](Summary of the chemical structures, pharmacology, relative potency and toxicology of the major synthetic opioids implicated in the ongoing opioid overdose epidemic; no discussion of hepatic injury).
- Grebely J, Feld JJ, Wyles D, Sulkowski M, Ni L, Llewellyn J, Mir HM, et al. Sofosbuvir-based direct-acting antiviral therapies for HCV in people receiving opioid substitution therapy: an analysis of phase 3 studies. Open Forum Infect Dis. 2018;5:ofy001. [PMC free article: PMC5808802] [PubMed: 29450210](Among 4743 patients enrolled in phase 3 trials of direct acting antiviral agents for HCV, 194 [4%] were on opioid substitution therapy including 75 on buprenorphine or buprenorphine/naloxone; the sustained virologic response rate was similar in those receiving buprenorphine [96%] to that in those who were not on any substitution therapy [97%] and adverse event rates were also similar).
- Once-monthly subcutaneous buprenorphine (Sublocade) for opioid use disorder. Med Lett Drugs Ther. 2018;60(1541):35–7. [PubMed: 29485976](Concise review of the mechanism of action, efficacy, safety and costs of a once-monthly formulation of buprenorphine given subcutaneously and providing plasma levels similar to those with sublingual buprenorphine, mentions that serum aminotransferase elevations are frequent with its use).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Opioids.[LiverTox: Clinical and Researc...]Review Opioids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers.[Psychopharmacology (Berl). 2000]Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers.Strain EC, Stoller K, Walsh SL, Bigelow GE. Psychopharmacology (Berl). 2000 Mar; 148(4):374-83.
- Review Sublingual buprenorphine-naloxone precipitated withdrawal-A case report with review of literature and clinical considerations.[Asian J Psychiatr. 2020]Review Sublingual buprenorphine-naloxone precipitated withdrawal-A case report with review of literature and clinical considerations.Bhatia G, Sarkar S. Asian J Psychiatr. 2020 Oct; 53:102121. Epub 2020 May 16.
- Effects of a higher-bioavailability buprenorphine/naloxone sublingual tablet versus buprenorphine/naloxone film for the treatment of opioid dependence during induction and stabilization: a multicenter, randomized trial.[Clin Ther. 2015]Effects of a higher-bioavailability buprenorphine/naloxone sublingual tablet versus buprenorphine/naloxone film for the treatment of opioid dependence during induction and stabilization: a multicenter, randomized trial.Gunderson EW, Hjelmström P, Sumner M, 006 Study Investigators. Clin Ther. 2015 Oct 1; 37(10):2244-55. Epub 2015 Sep 26.
- Review New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone.[Subst Abuse Rehabil. 2015]Review New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone.Soyka M. Subst Abuse Rehabil. 2015; 6:1-14. Epub 2015 Jan 6.
- Buprenorphine - LiverToxBuprenorphine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...