NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Brompheniramine and chlorpheniramine maleate are first generation antihistamines that are widely used to treat symptoms of allergic rhinitis and the common cold. Clinically apparent liver injury from brompheniramine or chlorpheniramine must be exceeding rare, if it occurs at all.
Background
Brompheniramine (brome" fen ir' a meen) and chlorpheniramine (klor" fen ir' a meen) are first generation antihistamines that are used widely in the therapy of the symptoms of sneezing, cough, runny note, watery eyes and itching. They are similar in chemical structure and constitute the alkylamine class of antihistamines. They are probably the most commonly used over-the-counter antihistamines, being present alone or in combination with other agents in more than 1000 products used for the symptoms of the common cold, sinusitis, urticaria and hay fever. Both agents are also available as their dextrorotatory isomers, dexbrompheniramine and dexchlorpheniramine, which have similar profiles of action and side effects. Common brand name preparations include Chlor-Trimeton, Dimetane, Drixoral and Durahist, and most are available without a prescription and in combination with sympathomimetic agents (such as pseudoephedrine or phenylephrine) or analgesics or both. The typical oral dose in adults is 2 to 4 mg three or four times a day. Common side effects include sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Likelihood scores: E (unlikely to be a cause of clinically apparent liver injury).
Hepatotoxicity
Despite widespread use, the first generation antihistamines such as brompheniramine and chlorpheniramine have rarely been linked to liver test abnormalities or to clinically apparent liver injury. A single case report of clinically apparent liver injury with jaundice attributed to dexchlorpheniramine was reported from France. The time to onset was 10 days, and the clinical presentation resembled acute viral hepatitis, with marked elevations in serum aminotransferase levels and jaundice. Recovery was rapid and complete, but jaundice and hepatitis recurred within 10 days of restarting. Immunoallergic features (rash, fever, eosinophilia) were absent as were autoantibodies. Interestingly, the patient tolerated other antihistamines (cetirizine) without difficulty.
Mechanism of Injury
The reason for the relative safety of brompheniramine and chlorpheniramine may be due to low daily dose and limited duration of treatment. The rare instances of hepatic injury associated with dexchlorpheniramine may relate to hypersensitivity.
Outcome and Management
Hepatic injury due to first generation antihistamines is very rare and most cases have been mild-to-moderate in severity and self-limited. Interestingly, recurrence of injury has been reported with reexposure to the same agent (dexchlorpheniramine, cetirizine, cyclizine, cyproheptadine), but not with switching to an unrelated antihistamine.
References on the safety and potential hepatotoxicity of antihistamines are given together after the Overview section on Antihistamines.
Drug Class: Antihistamines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Brompheniramine/Dexbrompheniramine – Generic, Ala-Hist®/Generic, Ala-Hist IR®
Chlorpheniramine/Dexchlorpheniramine – Generic, Chlor-Trimeton®/Generic
DRUG CLASS
Antihistamines
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Brompheniramine | 86-22-6 | C16-H19-Br-N2 |
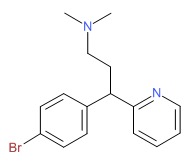 |
| Chlorpheniramine | 132-22-9 | C16-H19-Cl-N2 |
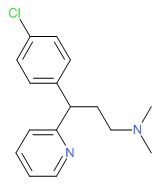 |
| Dexbrompheniramine | 132-21-8 | C16-H19-Br-N2 |
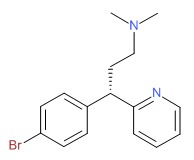 |
| Dexchlorpheniramine | 25523-97-1 | C16-H19-Cl-N2 |
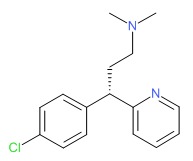 |
- Parabromdylamine maleate, chlorprophenpyridamine maleate, and tripelennamine hydrochloride in chronic allergic rhinitis.[J Allergy. 1959]Parabromdylamine maleate, chlorprophenpyridamine maleate, and tripelennamine hydrochloride in chronic allergic rhinitis.MACLAREN WR. J Allergy. 1959 May-Jun; 30(3):235-40.
- Affinities of brompheniramine, chlorpheniramine, and terfenadine at the five human muscarinic cholinergic receptor subtypes.[Pharmacotherapy. 1999]Affinities of brompheniramine, chlorpheniramine, and terfenadine at the five human muscarinic cholinergic receptor subtypes.Yasuda SU, Yasuda RP. Pharmacotherapy. 1999 Apr; 19(4):447-51.
- Brompheniramine maleate: a double-blind, placebo-controlled comparison with terfenadine for symptoms of allergic rhinitis.[Am J Rhinol. 1998]Brompheniramine maleate: a double-blind, placebo-controlled comparison with terfenadine for symptoms of allergic rhinitis.Thoden WR, Druce HM, Furey SA, Lockhart EA, Ratner P, Hampel FC, van Bavel J. Am J Rhinol. 1998 Jul-Aug; 12(4):293-9.
- Review First do no harm: managing antihistamine impairment in patients with allergic rhinitis.[J Allergy Clin Immunol. 2003]Review First do no harm: managing antihistamine impairment in patients with allergic rhinitis.Casale TB, Blaiss MS, Gelfand E, Gilmore T, Harvey PD, Hindmarch I, Simons FE, Spangler DL, Szefler SJ, Terndrup TE, et al. J Allergy Clin Immunol. 2003 May; 111(5):S835-42.
- Review Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines).[Clin Pharmacokinet. 1985]Review Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines).Paton DM, Webster DR. Clin Pharmacokinet. 1985 Nov-Dec; 10(6):477-97.
- Brompheniramine - LiverToxBrompheniramine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...