NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Bisacodyl is commonly used, over-the-counter laxative used to treat constipation or bowel irregularity. Bisacodyl has not been associated with serum enzyme elevations during therapy or with clinically apparent liver injury with jaundice.
Background
Bisacodyl (bis ak’ oh dil) is a mild laxative that is available over-the-counter and is commonly used to treat mild constipation and bowel irregularity. Bisacodyl is believed to act by direct stimulation of intestinal peristalsis. Bisacodyl is a diphenylmethane derivative and is structurally similar to phenolphthalein. It is administered in an enteric coated form and is minimally absorbed, acting locally on the large intestine. Bisacodyl has been in general use since the 1950s and is available in multiple forms including tablets of 5 mg, suppositories of 10 mg and as a liquid solution for oral use generically and under multiple trade names such as Dulcolax, Fleet’s enema, Correctal and Carter’s Little Pills. Bisacodyl is often used for bowel cleansing before operations or colonoscopy. Common side effects include abdominal pain, bloating and diarrhea.
Hepatotoxicity
Bisacodyl has not been associated with serum enzyme elevations during therapy or with instances of clinically apparent liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Bisacodyl is minimally absorbed and its metabolism has not been well characterized, but it is given in low doses only and generally for short periods of time.
Drug Class: Gastrointestinal Agents, Cathartics and Laxatives
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bisacodyl – Generic, Dulcolax®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bisacodyl | 603-50-9 | C22-H19-N-O4 |
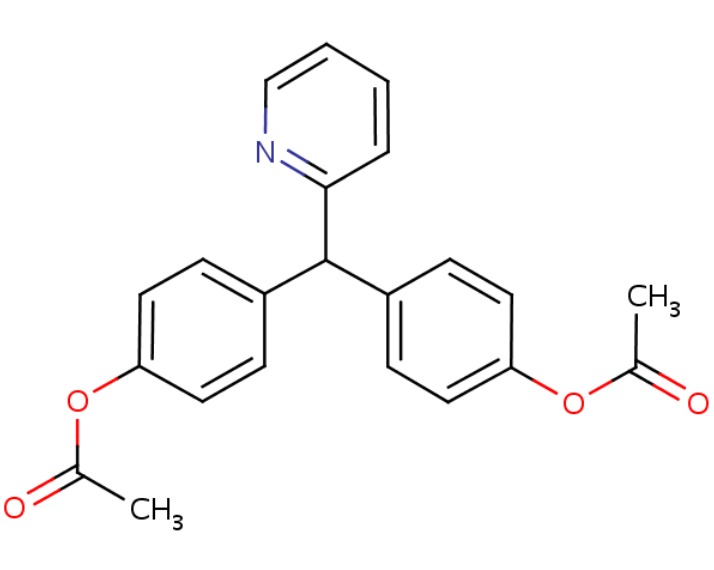 |
ANNOTATED BIBLIOGRAPHY
References updated: 29 September 2017
- Zimmerman HJ. Antiemetic and prokinetic compounds. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721.(Expert review of hepatotoxicity published in 1999 does not discuss bisacodyl).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-50.(Textbook of pharmacology and therapeutics).
- Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies or chronic constipation: systematic review. Am J Gastroenterol 2005; 100: 936-71. [PubMed: 15784043](Systematic review of the literature for controlled trials of traditional therapies for constipation reported "a paucity of trials for many commonly used agents", including bisacodyl and docusate).
- Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Aliment Pharmacol Ther 2006; 23: 1479-88. [PubMed: 16669963](Among 55 patients with idiopathic constipation treated with bisacodyl [10 mg once daily] or placebo for 3 days, there were no differences in side effects or "clinically meaningful differences" in serum chemistry results between the bisacodyl and placebo groups).
- Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Comparison of bisacodyl and sodium picosulphate in the treatment of chronic constipation. Curr Med Res Opin 2007; 23: 691-9. [PubMed: 17407625](Among 144 patients with constipation treated with bisacodyl or sodium picosulphate for 4 weeks, there was improvement in symptoms of constipation in both groups and side effects included abdominal pain and diarrhea; there were no significant changes in laboratory values including electrolytes and serum bilirubin).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no cases were attributed to laxatives).
- Mueller-Lissner SA, Wald A. Constipation in adults. Clin Evid (Online) 2010 Jul 5; 2010. pii: 0413. [PMC free article: PMC3217654] [PubMed: 21418672](Review of evidence of efficacy of conventional therapies of constipation).
- Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol 2011; 9: 577-83. [PubMed: 21440672](Among 368 patients with constipation treated with bisacodyl [10 mg] or placebo once daily for 4 weeks, numbers of bowel movements increased more in the bisacodyl [from 1.1 to 5.2 weekly] than the placebo recipients [1.1 to 1.9]; side effects included diarrhea [52% vs 1.7%] and abdominal pain [25% vs 2.5%], ALT levels were not determined and there were no serious adverse events attributed to bisacodyl).
- Gerard DP, Holden JL, Foster DB, Raiser MW. Randomized trial of gatorade/polyethylene glycol with or without bisacodyl and NuLYTELY for colonoscopy preparation. Clin Transl Gastroenterol 2012; 3: e16. [PMC free article: PMC3391000] [PubMed: 23238266](Among 600 patients scheduled for colonoscopy and undergoing bowel preparation with polyethylene glycol with or without bisacodyl and "NuLytely", patients receiving bisacodyl had more side effects [mostly abdominal bloating] without a significantly better colon preparation).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to laxatives).
- Müller-Lissner S. Pharmacokinetic and pharmacodynamic considerations for the current chronic constipation treatments. Expert Opin Drug Metab Toxicol 2013; 9: 391-401. [PubMed: 23425050](Review of the pharmacology, efficacy and safety of medications for constipation mentions that bisacodyl is minimally absorbed and is generally accepted as being safe).
- Kim HJ, Lee JH, Park HJ, Cho SH, Cho S, Kim WS. Monitoring of 29 weight loss compounds in foods and dietary supplements by LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2014; 31: 777-83. [PubMed: 24499058](Among 188 samples of 29 weight loss compounds sold as dietary supplements in Korea, 62 [33%] were found to be adulterated with conventional medications, including 7.1% with detectable levels of bisacodyl).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to bisacodyl or other laxatives).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- The concomitant use of an osmotic laxative, magnesium sulphate, and a stimulant laxative, bisacodyl, does not enhance the laxative effect.[Eur J Pharm Sci. 2012]The concomitant use of an osmotic laxative, magnesium sulphate, and a stimulant laxative, bisacodyl, does not enhance the laxative effect.Ikarashi N, Mimura A, Kon R, Iizasa T, Omodaka M, Nagoya C, Ishii M, Toda T, Ochiai W, Sugiyama K. Eur J Pharm Sci. 2012 Jan 23; 45(1-2):73-8. Epub 2011 Nov 9.
- Review Docusate.[LiverTox: Clinical and Researc...]Review Docusate.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Bisacodyl: A review of pharmacology and clinical evidence to guide use in clinical practice in patients with constipation.[Neurogastroenterol Motil. 2021]Review Bisacodyl: A review of pharmacology and clinical evidence to guide use in clinical practice in patients with constipation.Corsetti M, Landes S, Lange R. Neurogastroenterol Motil. 2021 Oct; 33(10):e14123. Epub 2021 Mar 9.
- Duodenal and proximal jejunal motility inhibition associated with bisacodyl-induced colonic high-amplitude propagating contractions.[Am J Physiol Gastrointest Live...]Duodenal and proximal jejunal motility inhibition associated with bisacodyl-induced colonic high-amplitude propagating contractions.Dinning PG, Wiklendt L, Costa M, Brookes SJH, Amicangelo M, Whitter L, Nurko S. Am J Physiol Gastrointest Liver Physiol. 2021 Sep 1; 321(3):G325-G334. Epub 2021 Jul 7.
- Review Management of faecal incontinence and constipation in adults with central neurological diseases.[Cochrane Database Syst Rev. 2014]Review Management of faecal incontinence and constipation in adults with central neurological diseases.Coggrave M, Norton C, Cody JD. Cochrane Database Syst Rev. 2014 Jan 13; 2014(1):CD002115. Epub 2014 Jan 13.
- Bisacodyl - LiverToxBisacodyl - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...