NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Berberine is a quaternary ammonia compound found in many botanical products, including goldenseal, barberry and Oregon grape, which is used for its purported antioxidant and antimicrobial properties for a host of conditions, including obesity, diabetes, hyperlipidemia, heart failure, H. pylori infection and colonic adenoma prevention. Berberine has not been linked to serum aminotransferase elevations during therapy nor to instances of clinically apparent liver injury.
Background
Berberine is an isoquinoline alkaloid that is found in many plants and is purported to have antioxidant, antimicrobial, antiinflammatory and antineoplastic activities. It has been used in Ayurvedic as well as traditional Chinese medicine for a wide variety of conditions, including infections, diarrhea, bronchitis and digestive complaints. Berberine is found in many plants and in particularly high concentrations in Hydrastis canadensis (goldenseal), Coptis chinensis (coptis or goldenthread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry) and Berberis aristata (tree turmeric). Berberine has been extensively evaluated in vitro and in vivo and demonstrates antioxidant, antiproliferative, and antimicrobial activities. It has been reported to be beneficial in alleviating symptoms of arthritis, in improving glucose control and insulin resistance, in improving hyperlipidemia and lowering blood pressure. Nevertheless, berberine has not been approved for any of these uses in the United States. Berberine is widely available over-the-counter in varying concentrations alone and combined with other herbs and nutritional substances in many multiingredient dietary supplements. The usual recommended dose of the herbal product is 250 to 500 mg two or three times daily. Side effects are few and largely mild and transient gastrointestinal symptoms of nausea, abdominal bloating, diarrhea or constipation. In most controlled studies, adverse events were no more frequent with berberine than with placebo and serious adverse events have been rare.
Hepatotoxicity
Berberine has not been linked to serum enzyme elevations during therapy, although there have been few prospective studies in humans that have reported on its effects on laboratory test results in any detail. In published trials, berberine has appeared to be well tolerated with only minor and few adverse effects which have been similar in frequency among persons receiving placebo. Despite wide scale use as an herbal supplement, berberine has not been linked to published instances of clinically apparent liver injury. The frequency of hypersensitivity reactions to berberine is also not known.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Other Names: Goldenseal, Oregon grape, Tree turmeric.
Drug Class: Herbal and Dietary Supplements
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Berberine – Generic
DRUG CLASS
Herbal and Dietary Supplements
SUMMARY INFORMATION
Fact Sheet at MedlinePlus, NLM
Fact Sheet at National Center for Complementary and Integrative Health, NIH [Goldenseal]
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Berberine | 2086-83-1 | C20-H18-N-O4 |
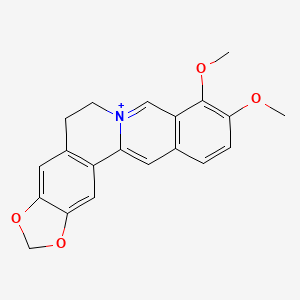
|
ANNOTATED BIBLIOGRAPHY
References updated: 06 October 2020
Abbreviations: HDS, herbal and dietary supplements.
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott,1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; several herbal medications linked to liver injury are discussed, but berberine is not mentioned).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbals does not mention berberine or goldenseal).
- PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007.(Compilation of short monographs on herbal medications and dietary supplements includes section on goldseal largely focused upon berberine).
- Zeng XH, Zeng XJ, Li YY. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:173–6. [PubMed: 12860219](Among 159 Chinese adults with congestive heart failure treated with conventional therapy with or without berberine [1.2 to 2 g/day] for a mean of 24 months, berberine therapy resulted in improvements in cardiac function and reduced mortality [9% vs 16%] with a “nonsignificant trend towards” an increase in side effects; no mention of ALT elevations or hepatotoxicity).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, including 7 [5%] for herbal medications, none were specifically attributed to a product containing berberine or goldenseal).
- Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7. [PMC free article: PMC2410097] [PubMed: 18442638](In 2 placebo controlled trials of berberine for 3 months in 36 and 48 patients with type 2 diabetes, therapy led to improvements in HbA1c and fasting blood glucose levels compared to placebo, and there were no “significant changes” in ALT levels or doubling of baseline ALT values in any patient).
- García-Cortés M, Borraz Y, Lucena MI, Peláez G, Salmerón J, Diago M, Martínez-Sierra MC, et al. Rev Esp Enferm Dig. 2008;100:688–95. [Liver injury induced by "natural remedies": an analysis of cases submitted to the Spanish Liver Toxicity Registry] Spanish. [PubMed: 19159172](Among 521 cases of drug induced liver injury submitted to Spanish registry, 13 [2%] were due to herbals, but none were attributed to berberine).
- Jacobsson I, Jönsson AK, Gerdén B, Hägg S. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039–47. [PubMed: 19650152](Review of 778 spontaneous reports of adverse reactions to herbals to Swedish Registry; no mention of berberine or goldenseal).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 [11%] were attributed to drug induced liver injury of which 12 [9%] were due to herbals, but none were attributed to berberine).
- Teschke R, Wolff A, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver Int. 2012;32:1543–56. [PubMed: 22928722](A systematic compilation of all publications on the hepatotoxicity of specific herbals identified 185 publications on 60 different herbs, herbal drugs and supplements, but berberine was not listed or mentioned).
- Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79:437–46. [PubMed: 23512497](Systematic review of the literature identified 11 randomized controlled trials of berberine [all from China] in 874 patients with hyperlipidemia that overall found significant declines in total and LDL cholesterol and triglycerides and a “remarkable” increase in HDL cholesterol compared to placebo, and “no serious adverse effects”, minor adverse events being largely gastrointestinal; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a prospective database between 2004 and 2012, HDS were implicated in 145 [16%], the single major herbal cause being green tea; no case was attributed to berberine [Navarro et al Hepatology 2014]).
- Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81. [PubMed: 25498346](Metaanalysis of 27 randomized controlled trials of berberine for type 2 diabetes and hypertension in 2569 patients found that its effects were similar to those of oral hypoglycemic agents and superior to placebo and that there were no serious adverse events, minor side effects being nausea, diarrhea, constipation, abdominal bloating and pain that were usually transient and did not require dose adjustment or discontinuation; no mention of hepatotoxicity).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: A tabular listing and clinical characteristics. Int J Mol Sci. 2016;17:537. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products, but does not mention or list berberine).
- Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 449-71. [PubMed: 27818322](Summary of the US regulations on safety and efficacy of herbal and dietary supplements).
- Brown AC. Liver toxicity related to herbs and dietary supplements: Online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017; 107(Pt A): 472-501. [PubMed: 27402097](Description of an online compendium of cases of liver toxicity attributed to HDS products, does not mention or list berberine).
- Guarino G, Strollo F, Carbone L, Della Corte T, Letizia M, Marino G, Gentile S. Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Bilybum marianum: a 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J Biol Regul Homeost Agents. 2017;31:495–502. [PubMed: 28685558](Among 136 obese subjects with type 2 diabetes and metabolic syndrome who were placed on a regimen of diet and exercise and treated with berberine [500 mg] combined with silymarin [105 mg] versus placebo twice daily for 6 months, improvements in HbA1c, LDL, HDL, total cholesterol, and triglyceride levels were greater with berberine therapy, and side effects were similar in both groups as well as laboratory values that included ALT levels that “changed similarly in both groups”).
- Zhang D, Ke L, Ni Z, Chen Y, Zhang LH, Zhu SH, Li CJ, Shang L, Liang J, Shi YQ. Berberine containing quadruple therapy for initial Helicobacter pylori eradication: An open-label randomized phase IV trial. Medicine (Baltimore). 2017;96:e7697. [PMC free article: PMC5556219] [PubMed: 28796053](Among 612 adult with H. pylori infection treated with clarithromycin, amoxicillin, and omeprazole with either berberine or bismuth for 14 days, eradication rates were similar [90% vs 86%] while adverse events were slightly higher with berberine [36% vs 29%], including nausea [12% vs 4%]; no mention of ALT elevations or hepatotoxicity).
- Chen YX, Gao QY, Zou TH, Wang BM, Liu SD, Sheng JQ, Ren JL, et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomized controlled study. Lancet Gastroenterol Hepatol. 2020;5:267–75. [PubMed: 31926918](Among 891 Chinese patients with adenomatous polyps on colonoscopy treated with either berberine or placebo for 2 years, recurrence of adenoma was less frequent with berberine [36% vs 47%] while there were no serious adverse events; no mention of ALT elevations or hepatotoxicity).
- Mandal SK, Maji AK, Mishra SK, Ishfaq PM, Devkota HP, Silva AS, Das N. Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues. Pharmacol Res. 2020;160:105085. [PubMed: 32683037](Review of the chemical ingredients, pharmacologic activities, clinical efficacy and safety of goldenseal extracts in the treatment of many conditions, including obesity, hyperlipidemia, diabetes, hypertension, bacterial, fungal and viral infections, cancer, arthritis, Alzheimer disease, renal and liver disease with short discussions of toxicity, which were largely limited to in vitro and in vivo studies; no mention of ALT elevations or hepatotoxicity in humans).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Berberine.[Altern Med Rev. 2000]Berberine.. Altern Med Rev. 2000 Apr; 5(2):175-7.
- Review Berberine and Its Role in Chronic Disease.[Adv Exp Med Biol. 2016]Review Berberine and Its Role in Chronic Disease.Cicero AF, Baggioni A. Adv Exp Med Biol. 2016; 928:27-45.
- Method validation for determination of alkaloid content in goldenseal root powder.[J AOAC Int. 2003]Method validation for determination of alkaloid content in goldenseal root powder.Weber HA, Zart MK, Hodges AE, White KD, Barnes SM, Moody LA, Clark AP, Harris RK, Overstreet JD, Smith CS. J AOAC Int. 2003 May-Jun; 86(3):476-83.
- Determination of hydrastine and berberine in goldenseal raw materials, extracts, and dietary supplements by high-performance liquid chromatography with UV: collaborative study.[J AOAC Int. 2008]Determination of hydrastine and berberine in goldenseal raw materials, extracts, and dietary supplements by high-performance liquid chromatography with UV: collaborative study.Brown PN, Roman MC. J AOAC Int. 2008 Jul-Aug; 91(4):694-701.
- Review Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues.[Pharmacol Res. 2020]Review Goldenseal (Hydrastis canadensis L.) and its active constituents: A critical review of their efficacy and toxicological issues.Mandal SK, Maji AK, Mishra SK, Ishfaq PM, Devkota HP, Silva AS, Das N. Pharmacol Res. 2020 Oct; 160:105085. Epub 2020 Jul 16.
- Berberine - LiverToxBerberine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...