NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Belinostat is an intravenously administered histone deacetylase inhibitor and antineoplastic agent that is approved for use in refractory or relapsed peripheral T cell lymphoma. Belinostat is associated with moderate rate of minor serum enzyme elevations during therapy and has been reported to cause clinically apparent fatal, acute liver injury.
Background
Belinostat (be lin' oh stat) is a parenterally administered small molecule inhibitor of histone deacetylase which acts by preventing removal of acetyl groups from histones. Accumulation of acetyl groups on histones causes cell cycle arrest and apoptotic cell death. Malignant cells and particularly malignant T cells are particularly sensitive to the effects of inhibition of histone deacetylases. In open label studies in patients with refractory or relapsed peripheral T cell lymphoma (PTCL), monotherapy with belinostat yielded an overall response rate of 26% and some responders had long term remissions and were able to undergo hematopoietic cell transplantation. Belinostat was approved for use in the United States in 2014 as monotherapy for refractory or relapsing peripheral T cell lymphoma. Belinostat is available as a lyophilized powder for reconstitution in 500 mg single use vials under the commercial name Beleodaq. The recommended dose is 1 gm/m2 given intravenously on days 1 to 5 in 21 day cycles, which are repeated until there is disease progression or unacceptable toxicity. Side effects are common, but usually mild-to-moderate in severity, and include nausea, fatigue, fever, anemia, neutropenia, thrombocytopenia, constipation, rash, edema, cough and pruritus. Side effects lead to early discontinuation in up to 20% of patients. Severe adverse events can include marked neutropenia, thrombocytopenia, serious infections, sepsis, tumor lysis syndrome and acute liver failure.
Hepatotoxicity
In clinical trials of belinostat in patients with PTCL, the rates of serum enzyme elevations during therapy were usually less than 5%, and were above 5 times the ULN in only 1% to 2% of patients. A single instance of severe acute liver injury leading to death from liver failure was reported in an open label trial of belinostat monotherapy in 120 patients with PTCL. The liver injury arose after 10 cycles of treatment and progressed despite drug discontinuation. Specific details were not provided. In another clinical trial, two cases of cholestatic liver injury were reported but without specific details. Thus, belinostat is considered to be a rare cause of acute liver injury but the timing of onset, associated features, clinical course and outcome have not been well defined.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The reason why belinostat causes serum enzyme elevations is not known, but may be a direct toxicity to hepatocytes caused by inhibition of histone deacetylase or other enzyme activities. Belinostat is metabolized in the liver by UGT1A1 and the cytochrome P450 isozymes CYP 2A6, 2C9 and 3A4, so that it may cause drug-drug interactions, and hepatotoxicity could be the result of production of a toxic or immunogenic intermediate. Finally, belinostat is a sulfonamide hydroxyamide and severe acute liver injury from belinostat might be the result of a sulfonamide-like hepatic reaction.
Outcome and Management
Serum enzyme elevations are uncommon during belinostat therapy and are rarely dose limiting. Belinostat should not be used with other agents with hepatotoxic potential. Furthermore, regular monitoring of liver tests with each course of therapy is recommended, with more frequent monitoring if serum aminotransferase values rise. Belinostat should be held if ALT or AST values rise above 5 times the ULN. Elevations of more than 20 times the ULN or appearance of jaundice or symptoms of liver injury should trigger permanent discontinuation. There is no known cross sensitivity to hepatic injury among the different histone deacetylase inhibitors. Because belinostat is a sulfonamide derivative, it should be used with caution in patients with a history of sulfa allergy.
Drug Class: Antineoplastic Agents, Histone Deacetylase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Belinostat – Beleodaq®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
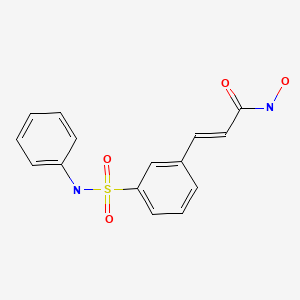
| Belinostat | 866323-14-0 | C15-H14-N2-O4-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 25 September 2020
- Abbreviation: PTCL, peripheral T-cell lymphoma.
- Zimmerman HJ. Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of histone deacetylase inhibitors).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; does not discuss belinostat).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Inhibitors of histone deacetylase. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, p. 1230.(Textbook of pharmacology and therapeutics).
- Mackay HJ, Hirte H, Colgan T, Covens A, MacAlpine K, Grenci P, Wang L, et al. Phase II trial of the histone deacetylase inhibitor belinostat in women with platinum resistant epithelial ovarian cancer and micropapillary (LMP) ovarian tumours. Eur J Cancer. 2010;46:1573–9. [PMC free article: PMC3244274] [PubMed: 20304628](Among 32 women with refractory ovarian carcinoma treated with belinostat, the overall response rate was 3% and common side effects included fatigue [69%], nausea [59%], diarrhea [28%], neuropathy [16%] and Alk P elevations [6%], but there were no cases of clinically apparent liver injury).
- Dizon DS, Blessing JA, Penson RT, Drake RD, Walker JL, Johnston CM, Disilvestro PA, et al. A phase II evaluation of belinostat and carboplatin in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:367–71. [PMC free article: PMC3330705] [PubMed: 22366594](Among 27 women with refractory ovarian carcinoma treated with belinostat and carboplatin, the overall response rate was 7% and severe side effects included neutropenia [22%], thrombocytopenia [15%] and vomiting [11%]; no mention of ALT elevations or hepatotoxicity).
- Cashen A, Juckett M, Jumonville A, Litzow M, Flynn PJ, Eckardt J, LaPlant B, et al. Phase II study of the histone deacetylase inhibitor belinostat (PXD101) for the treatment of myelodysplastic syndrome (MDS). Ann Hematol. 2012;91:33–8. [PMC free article: PMC3557843] [PubMed: 21538061](Among 21 patients with refractory myelodysplastic syndromes treated with belinostat, the overall response rate was 5% and 90% had severe cytopenias, 2 had cytokine release syndrome and 2 had QTc prolongations; no mention of ALT elevations or hepatotoxicity).
- Reimer P, Chawla S. Long-term complete remission with belinostat in a patient with chemotherapy refractory peripheral T-cell lymphoma. J Hematol Oncol. 2013;6:69. [PMC free article: PMC3846498] [PubMed: 24020452](73 year old woman with refractory PTCL had a complete response after 2 cycles of belinostat, which was then continued for 2 years with maintained response thereafter; side effects included cytopenias, 3 bouts of C. difficile colitis and 1 episode of pneumonia).
- Kirschbaum MH, Foon KA, Frankel P, Ruel C, Pulone B, Tuscano JM, Newman EM. A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: a California Cancer Consortium Study. Leuk Lymphoma. 2014;55:2301–4. [PMC free article: PMC4143479] [PubMed: 24369094](Among 12 patients with AML treated with belinostat, none had even a partial response and side effects included ALT elevations in 4 patients [33%], although all were transient and less than 5 times ULN).
- Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, Shpilberg O, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168:811–9. [PubMed: 25404094](Among 24 patients with refractory PTCL and 29 with cutaneous TCL treated with belinostat, overall response rates were 25% and 14% and adverse events occurred in 77%, including 2 patients with ALT or AST elevations above 5 times ULN, one of whom stopped therapy for this reason).
- O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33:2492–9. [PMC free article: PMC5087312] [PubMed: 26101246](Among 120 patients with refractory PTCL treated with belinostat for up to 2 years, the overall response rate was 26%, and side effects were common [97%] and included two cases of ALT elevations above 5 times ULN and one that led to hepatic failure shortly after starting cycle 10).
- Belinostat (Beleodaq) for peripheral T-Cell lymphoma. Med Lett Drugs Ther. 2015;57:e66–7. [PubMed: 25988963](Concise review of mechanism of action, efficacy, safety and cost of belinostat shortly after its approval for use in PTCL in the US, mentions that reported serious adverse events include fatal hepatotoxicity).
- Lee HZ, Kwitkowski VE, Del Valle PL, Ricci MS, Saber H, Habtemariam BA, Bullock J, et al. FDA approval: belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin Cancer Res. 2015;21:2666–70. [PubMed: 25802282](Summary of evidence that supported the accelerated approval of belinostat for PTCL, largely from the open label trial reported by O’Connor [2015] demonstrating a 26% overall response rate and acceptable toxicity; 4 patients [3%] developed tumor lysis syndrome and there were 9 deaths during the trial including one from hepatic failure that was the only one judged to be related to therapy).
- Lunning MA. Treatment of peripheral T-cell lymphoma: many shades of gray. Oncology (Williston Park). 2015;29:545–50. [PubMed: 26281838](Review of PTCL which represents 10% of cases of non-Hodgkin lymphoma and is often refractory to conventional cytotoxic therapies; recently 4 new agents have been approved for use in PTCL including pralatrexate [2009], romidepsin [2011], brentuximab vedotin [2011] and belinostat [2014]).
- Puvvada SD, Li H, Rimsza LM, Bernstein SH, Fisher RI, LeBlanc M, Schmelz M, Glinsmann-Gibson B, et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520. Leuk Lymphoma. 2016;57(10):2359–69. [PMC free article: PMC5140034] [PubMed: 26758422](Among 22 patients with refractory or relapsing B cell lymphoma treated with belinostat for up to two years, the overall response rate was only 10% and side effects were common, although usually tolerated; no mention of ALT elevations or hepatotoxicity).
- Moskowitz AJ, Horwitz SM. Targeting histone deacetylases in T-cell lymphoma. Leuk Lymphoma. 2017;58:1306–19. [PubMed: 27813438](Extensive review of the mechanism of action, classification, clinical efficacy and safety of histone deacetylase inhibitors in T-cell lymphomas, mentions that belinostat is a parenterally administered hydroxamate that is approved for use in refractory or relapsed peripheral T cell lymphoma, more common adverse effects of which are nausea, vomiting, fatigue, diarrhea, constipation, fever and dizziness;, no mention of ALT elevations or hepatotoxicity).
- Shah RR. Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Saf. 2019;42:235–45. [PubMed: 30649740](Review of the safety of histone deacetylase inhibitors approved for use in the US mentions that elevations in serum aminotransferase levels have been reported during therapy with romidepsin, panobinostat and belinostat but not with vorinostat, and there have been no reports of clinically apparent hepatotoxicity, except for a single case of hepatic failure arising during a clinical trial of belinostat [O’Connor 2015]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma.[Future Oncol. 2015]Review Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma.Rashidi A, Cashen AF. Future Oncol. 2015; 11(11):1659-64.
- Review Romidepsin.[LiverTox: Clinical and Researc...]Review Romidepsin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma.[J Oncol Pharm Pract. 2017]Review Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma.Campbell P, Thomas CM. J Oncol Pharm Pract. 2017 Mar; 23(2):143-147. Epub 2016 Jun 23.
- A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma.[Br J Haematol. 2015]A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma.Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, Shpilberg O, Lerner A, Belt RJ, Jacobsen ED, et al. Br J Haematol. 2015 Mar; 168(6):811-9. Epub 2014 Nov 17.
- Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study.[J Clin Oncol. 2015]Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study.O'Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S, et al. J Clin Oncol. 2015 Aug 10; 33(23):2492-9. Epub 2015 Jun 22.
- Belinostat - LiverToxBelinostat - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...