NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Azithromycin is a semisynthetic macrolide antibiotic which is commonly used for a wide variety of mild-to-moderate bacterial infections. Azithromycin has been linked to rare instances of acute liver injury.
Background
Azithromycin (ay zith" roe mye' sin) is a semisynthetic macrolide antibiotic used widely to treat mild-to-moderate bacterial infections caused by sensitive agents. Azithromycin, like other macrolide antibiotics such as erythromycin and clarithromycin, is bacteriostatic against many gram positive bacteria including many strains of streptococci, staphylococci, clostridia, corynebacteria, listeria, haemophilus sp., moxicella, and Neisseria meningitidis. Azithromycin is more active than erythromycin against several gram negative bacteria as well as Mycoplasma pneumonia, Helicobacter pylori, Toxoplasma gondii, cryptosporidia and several atypical mycobacteria. Macrolide antibiotics act by inhibiting protein synthesis of bacteria by binding to the 50S ribosomal element. Resistance occurs by several mechanisms. Azithromycin was approved for use in the United States in 1994 and currently it is the most commonly prescribed antibiotic in America. Typical indications are community acquired pneumonia, acute exacerbations of chronic bronchitis, sinusitis, pelvic inflammatory disease, urethritis and other infections caused by susceptible bacteria. Azithromycin is also used to treat disseminated mycobacterium avium complex infections. Azithromycin is available as tablets of 250 and 500 mg and as solutions and powders for suspension generically and under the name Zithromax. Azithromycin is typically given in once daily doses for 5 to 7 days. Chronic use of azithromycin is used to treat atypical mycobacterial infections and as prophylaxis against common bacterial infections in highly susceptible persons (with cystic fibrosis, chronic granulomatous disease, or bronchiectasis). Parenteral azithromycin is typically given in doses of 500 mg iv daily for the first few days of therapy in moderate-to-severe infections. Azithromycin is generally well tolerated, but side effects can include nausea, abdominal pain, diarrhea, dyspepsia, headache, dizziness, angioedema and rash. Severe adverse reactions include infantile pyloric stenosis, Clostridia difficile diarrhea, QTc prolongation, hepatotoxicity and severe hypersensitivity reactions including Stevens Johnson syndrome and toxic epidermal necrolysis.
Hepatotoxicity
Like other macrolide antibiotics, azithromycin has been linked to a low rate of acute, transient and asymptomatic elevation in serum aminotransferases which occurs in 1% to 2% of patients treated for short periods, and a somewhat higher proportion of patients given azithromycin long term.
Azithromycin can also rarely cause clinically apparent liver injury. Because azithromycin has become so commonly used, it has also become one of the more common causes of drug induced liver injury. The typical liver injury caused by azithromycin resembles that described with other macrolides and is a self-limited, cholestatic hepatitis, arising within 1 to 3 weeks of starting treatment (Case 1). It occasionally arises after azithromycin is stopped and can occur even after a short, 2 or 3 day course. Typical symptoms are fatigue, jaundice, abdominal pain and pruritus. Fever and eosinophilia may also be present, but immunoallergic features are usually not prominent. This form of liver injury from azithromycin is usually benign, but in some instances is associated with prolonged jaundice and persistence of liver test abnormalities for 6 months or more. Liver histology in these cases generally demonstrates bile duct loss, which if severe, can result in vanishing bile duct syndrome and chronic cholestatic liver failure, ultimately requiring liver transplantation. Other instances of bile duct loss and prolonged cholestatic hepatitis ultimately resolve, but may be marked by persistent serum alkaline phosphatase elevations of uncertain clinical significance.
Azithromycin can also cause hepatocellular injury with symptoms and jaundice. In these cases, the latency is typically short and may be 1 to 3 days only (Case 2). Serum aminotransferase levels are markedly elevated and alkaline phosphatase values are usually less than twice the upper limit of normal, although they may rise to higher levels with time. The hepatocellular forms of liver injury from azithromycin can be severe and lead to acute liver failure and death or need for emergency liver transplantation. However, in most cases, recovery occurs within 4 to 8 weeks.
Azithromycin has also been associated with severe cutaneous reactions such as erythema multiforme, drug reaction with eosinophilia and systemic signs (DRESS) syndrome, Stevens Johnson syndrome (SJS) and toxic epidermal necrosis (TEN). These severe cutaneous reactions are often associated with some degree of liver injury and may be accompanied by clinically apparent injury with jaundice, usually with a cholestatic pattern. However, the severe cutaneous reactions usually overshadow the liver injury.
In cell culture, azithromycin exhibits some degree of antiviral activity and was shown to inhibit the Severe Acute Respiratory Syndrome, coronavirus type 2 (SARS-CoV-2), the cause of the pandemic of severe COVID-19 pneumonia that arose in 2019 and led to more than 3 million deaths worldwide. In view of the antiviral activity as well as antiinflammatory actions of azithromycin, it was repurposed as a potential therapy of COVID-19. Despite early promising reports, subsequent randomized controlled trials found that it had no benefit in either prevention of infection or amelioration of the course of COVID-19 illness. An emergency use authorization granted to azithromycin as therapy of COVID-19 in early 2020 was later withdrawn.
Likelihood score: A (well known but rare cause of clinically apparent liver injury).
Histopathology
Azithromycin hepatotoxicity is typically associated histologically with a cholestatic hepatitis similar to what is described with erythromycin induced liver injury. Scattered bile casts are found in canaliculi with modest parenchymal inflammation and necrosis. Portal tracts often have more pronounced inflammation characterized by mononuclear cells and eosinophils. The cholestasis can be profound and prolonged, and azithromycin is a well known cause of vanishing bile duct syndrome. Less common is hepatocellular injury, a pattern seen most commonly with reexposure, with a short latency to onset and biopsy taken early in the course of injury.
CHOLESTATIC HEPATITIS with Bile Plugs and Scant Parenchymal Inflammation
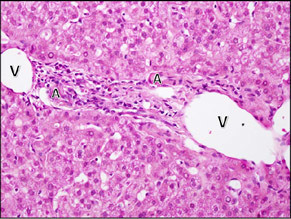
| Cholestasis with duct paucity. In this case, more than 90% of the portal areas lacked a bile duct. In this portal area, there is mild inflammation with rare eosinophils. The artery (A) and vein (V) can be seen, but there is no duct and no ductular reaction. |
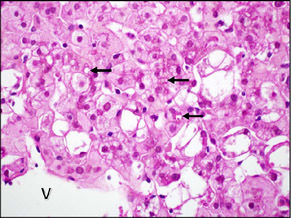
| Small bile plugs can be seen in dilated canaliculi in zone 3 (arrows). There was very little parenchymal inflammation in this case and the hepatocytes show cytoplasmic glycogenosis. The central vein (V) is the bottom left corner. |
VANISHING BILE DUCT SYNDROME
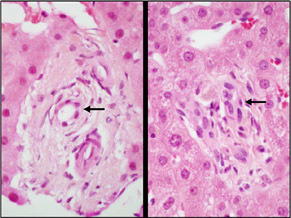
| Bile duct injury was seen in most of the portal areas, although there was very little inflammation. The bile ducts (arrows) show different kinds of injury. In the portal area on the left, the duct epithelium is attenuated, and there is a suggestion of periductal fibrosis. In the portal area on the right, the bile duct epithelium shows reactive changes, with enlarged, irregularly spaced nuclei that have open chromatin. |
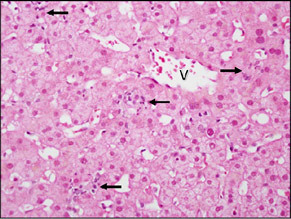
| There was mild predominantly lobular inflammation with scattered small foci of lymphocytes and macrophages (arrows). Rare apoptotic hepatocytes were also present (not shown). The central vein is indicated (V). |
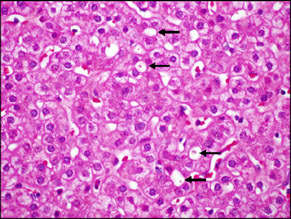
| This patient had prolonged cholestasis and a biopsy was performed almost a year after the onset of the injury. There was almost no inflammation in the portal areas or parenchyma and the bile ducts were intact. However, bile plugs could still be found with dilated canaliculi in zone 3 (arrows). |
Mechanism of Liver Injury
The cause of the idiosyncratic liver injury due to azithromycin is unknown, but the rapidity of onset suggests hypersensitivity as a cause.
Outcome and Management
The minor serum aminotransferase elevations that appear during therapy with azithromycin are usually benign, asymptomatic and resolve rapidly whether or not azithromycin is stopped. The acute hepatic injury with jaundice, however, can be prolonged and troublesome and lead to loss of intrahepatic bile ducts and vanishing bile duct syndrome. The injury and jaundice may arise after azithromycin is stopped, but appearance of jaundice in someone on azithromycin should lead to its prompt discontinuation. Rare instances of acute liver failure and fatality from azithromycin induced liver disease have been reported. Persons with preexisting, underlying chronic liver disease may be more susceptible to severe outcomes. There is likely to be cross reactivity in hepatic injury with other macrolide antibiotics, but this has not been well documented.
Drug Class: Antiinfective Agents, Macrolide Antibiotics
CASE REPORTS
Case 1. Cholestatic hepatitis after azithromycin.(1)
A 69 year old man with community acquired pneumonia was treated with azithromycin (500 mg daily) for 3 days and developed dark urine and jaundice 3 days later. Laboratory tests showed marked elevations in alkaline phosphatase and bilirubin with modest increases in ALT and AST levels (Table). He tested negative for markers of hepatitis A, B and C infection, and liver ultrasound was normal. He was given ceftriaxone for his unresolved bronchopneumonia and recovered within the next few weeks.
Key Points
| Medication: | Azithromycin (500 mg daily for 3 days) |
|---|---|
| Pattern: | Cholestatic (R=0.4) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 1 week |
| Recovery: | Complete within one month |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| Dark urine and jaundice 3 days after stopping azithromycin | |||||
| 1 week | 4 days | 49 | 890 | 9.1 | GGT 131 |
| 2 weeks | 11 days | 90 | 1200 | 15.2 | |
| 3 weeks | 18 days | 156 | 450 | 6.0 | |
| 4 weeks | 25 days | 30 | 210 | 1.2 | GGT 45 |
| Normal Values | <40 | <280 | <1.2 | ||
Comment
This was an early case report of azithromycin hepatotoxicity and clearly demonstrated its typical clinical pattern of abrupt onset of illness within a week of starting the medication—and actually a few days after stopping it. The cholestatic pattern of liver enzymes and jaundice are typical (but not invariable) of azithromycin hepatotoxicity. There was no mention of signs of hypersensitivity such as fever, rash, arthralgias or eosinophilia, although the concurrent pneumonia may have confounded the clinical picture. Recovery was reasonably rapid, but the benign outcome may have been because azithromycin was given for 3 days only. This patient should be advised against future exposure to azithromycin, but there is little information about his risks of liver injury with other macrolide antibiotics (such as erythromycin or clarithromycin).
Case 2. Hepatocellular liver injury due to azithromycin.(2)
A 69 year old woman developed weakness, anorexia, nausea, diarrhea, pruritus and jaundice 4 days after starting oral azithromycin (1000 mg initially, then 500 mg daily) for suspected acute bronchitis. She had no history of liver disease, did not drink alcohol and had no risk factors for viral hepatitis. Her past medical history included hypertension, dyslipidemia, a 50 pack year history of cigarette smoking, chronic obstructive pulmonary disease, hypothyroidism and depression for which she was taking lisinopril, simvastatin, aspirin, loratadine, albuterol by inhaler, levothyroxine and fluoxetine. She was also increasingly symptomatic from her acute pulmonary infection. Physical examination revealed jaundice but no fever, rash or organomegaly. She was dyspneic and had basilar inspiratory crackles and signs of consolidation. Laboratory tests showed bilirubin of 2.0 (direct 0.9) mg/dL, ALT 1065 U/L, AST 2001 U/L, Alk P 125 U/L and INR of 2.3 (Table). Tests for acute hepatitis A, B and C (including HCV RNA) were negative. ANA was negative, but smooth muscle antibody was reactive. A chest CT showed bibasilar pulmonary consolidations. She was admitted to the hospital, azithromycin was held and levofloxacin started. The following day she had a respiratory arrest, but was successfully resustained and placed on mechanical ventilation. Ultrasound of the abdomen showed no evidence of biliary obstruction although there was biliary sludge and gallbladder wall thickening. Over the first week of admission her serum enzymes remained high and serum bilirubin climbed to 18.5 mg/dL. However, her pulmonary status improved, she was successfully extubated and was eventually discharged on her previous medications without antibiotics and with improving serum enzymes. When seen in the outpatient clinic 2 and 5 months later, her liver tests were normal.
Key Points
| Medication: | Azithromycin (500-1000 mg daily orally for 4 days) |
|---|---|
| Pattern: | Hepatocellular (R=20) |
| Severity: | 4+ (jaundice, hospitalization, prolonged INR) |
| Latency: | 4 days |
| Recovery: | Complete within two months |
| Other medications: | Chronically: lisinopril, simvastatin, aspirin, albuterol, loratadine, levothyroxine, fluoxetine |
Laboratory Values
| Time After Starting | Time After Starting | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| Fatigue, weakness, nausea and jaundice 4 days after starting azithromycin | |||||
| 4 days | 0 | 1065 | 125 | 2.0 | Admission, INR 2.3 |
| 5 days | 1 days | 4068 | 140 | 2.9 | Respiratory arrest |
| 7 days | 3 days | 1101 | 134 | 11.6 | |
| 14 days | 10 days | 198 | 89 | 15.4 | |
| 2 months | 35 | 89 | 1.6 | Outpatient | |
| 5 months | 19 | 73 | 0.4 | ||
| Normal Values | <54 | <126 | <1.2 | ||
Comment
The rapidity of onset of injury after starting azithromycin suggests previous exposure either to azithromycin specifically or to another macrolide. The initial injury was distinctly hepatocellular, with ALT levels 20 fold elevated and alkaline phosphatase at the upper limit of the normal range. The clinical course was complicated by a fairly severe pneumonitis in the setting of chronic obstructive pulmonary disease that led to a respiratory arrest, which may have accounted for the second rise in ALT levels and worsening of jaundice for 7 to 10 days after stopping the implicated antibiotic. Several of the other medications she was taking have a potential to cause acute liver injury, but all were restarted without recurrence. Thus, while atypical in some regards, the timing and course of the liver injury make azithromycin the most likely cause.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Azithromycin — Generic, Zithromax®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Azithromycin Dihydrate | 117772-70-0 | C38-H72-N2-O12.2H2O |

|
Azithromycin is a macrolide antibiotic and semisynthetic derivative of erythromycin with a methyl-substituted nitrogen atom in the large lactone ring. It is orally bioavailable, has a prolonged half-life (allowing for once daily administration), and exhibits excellent tissue penetration and distribution.
CITED REFERENCES
- 1.
- Longo G, Valenti C, Gandini G, Ferrara L, Bertesi M, Emilia G. Azithromycin-induced intrahepatic cholestasis. Am J Med. 1997;102:217–8. [PubMed: 9217574]
- 2.
- Lockwood AM, Cole S, Rabinovich M. Azithromycin-induced liver injury. Am J Health Syst Pharm. 2010;67:810–4. [PubMed: 20479103]
ANNOTATED BIBLIOGRAPHY
References updated: 20 April 2021
- Zimmerman HJ. Erythromycins. In, Zimmerman HJ. Hepatotoxicity: The adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 1999, pp. 594-5.(Expert review of erythromycin and liver injury published in 1999; mentions clarithromycin, but not azithromycin).
- Moseley RH. Macrolide antibiotics. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced Liver Disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 466-7.(Expert review of macrolide antibiotic induced liver injury; mentions that cholestatic injury has been reported with azithromycin).
- MacDougall C, Chambers HF. Macrolides and ketolides. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1529-34.(Textbook of pharmacology and therapeutics).
- Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, et al. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54. [PMC free article: PMC175833] [PubMed: 2830841](Preclinical pharmacology of azithromycin, its advantages over erythromycin are that it has a longer half-life and excellent tissue distribution).
- Vial T, Biour M, Descotes J, Trepo C. Antibiotic-associated hepatitis: update from 1990. Ann Pharmacother. 1997;31:204–20. [PubMed: 9034423](Extensive review including discussion of three macrolides [erythromycin, clarithromycin and azithromycin], ALT elevations occur in 0.4-1.2% of treated patients but similar rates found in placebo controls; rare instances of cholestatic hepatitis have been reported with all three macrolide antibiotics).
- Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91(3A):40S–45S. [PubMed: 1656742](Review of side effects of azithromycin in 3995 patients treated for 1-5 days: side effects occurred in 12%, most commonly gastrointestinal symptoms; transient increases in ALT in 1.7% and AST in 1.5%, but similar or higher rates [2.5% and 1.0%] with comparative drugs, 2 patients withdrawn because of ALT elevations, but no mention of hepatitis or jaundice).
- Longo G, Valenti C, Gandini G, Ferrara L, Bertesi M, Emilia G. Azithromycin-induced intrahepatic cholestasis. Am J Med. 1997;102:217–8. [PubMed: 9217574](69 year old man developed jaundice 3 days after a 3 day course of azithromycin [peak bilirubin 16.2 mg/dL, ALT 156 U/L, Alk P 1200 U/L], resolving within 2 weeks of stopping: Case 1).
- Macaigne G, Mokbel M, Marty O, De La Lande P, Mallet L. Gastroenterol Clin Biol. 2000;24:969–70. [Acute pseudoangiocholitic hepatitis probably induced by azithromycin] French. [PubMed: 11084438](55 year old woman developed fever, jaundice and right upper quadrant pain 18 days after starting a 5 day course of azithromycin [bilirubin of 8.9 mg/dL ALT 2.8 times and Alk P 3.5 times ULN], resolving within 3 weeks of stopping except for residual mild Alk P elevations).
- Cascaval RI, Lancaster DJ. Hypersensitivity syndrome associated with azithromycin. Am J Med. 2001;110:330–1. [PubMed: 11247598](79 year old man developed rash, fever and eosinophilia 10 days after starting a 5 day course of azithromycin, with renal failure and obtundation [bilirubin not given, ALT 210 U/L, Alk P 1512 U/L], resolving rapidly but scant data given).
- Chandrupatla S, Demetris AJ, Rabinovitz M. Azithromycin-induced intrahepatic cholestasis. Dig Dis Sci. 2002;47:2186–8. [PubMed: 12395890](72 year old man developed jaundice 12 days after starting a 5 day course of azithromycin [bilirubin 3.3 mg/dL, ALT 156 U/L, Alk P 349 U/L], resolving within next month).
- Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet. 2002;360(9338):978–84. [PubMed: 12383667](Controlled, crossover trial of azithromycin vs placebo for 6 months in 41 children with cystic fibrosis; one child developed ALT elevations >3 times ULN, improving upon stopping).
- Suriawinata A, Min AD. A. 33-year-old woman with jaundice after azithromycin use. Semin Liver Dis. 2002;22:207–10. [PubMed: 12016551](33 year old woman developed jaundice 4 days after completing a 5 day course of azithromycin and 1 day after starting erythromycin [bilirubin 11.3 mg/dL, ALT 583 U/L, Alk P 228 U/L], with severe pruritus, resolving within 2 months of onset).
- Sopena N, Martínez-Vázquez C, Rodríguez-Suárez JR, Segura F, Valencia A, Sabrià M. Comparative study of the efficacy and tolerance of azithromycin versus clarithromycin in the treatment of community-acquired pneumonia in adults. J Chemother. 2004;16:102–3. [PubMed: 15078008](Comparison of 3 days of azithromycin vs 10-14 days of clarithromycin in 70 adult patients with pneumonia showing similar efficacy [>90%] and frequency of side effects [26.5% vs 25%], with no mention of hepatic injury).
- Baciewicz AM, Al-Nimr A, Whelan P. Azithromycin-induced hepatoxicity. Am J Med. 2005;118:1438–9. [PubMed: 16378809](75 year old woman developed jaundice 2 days after completing a 3 day course of azithromycin [peak bilirubin 30.7 mg/dL, ALT 909 U/L, Alk P 357 U/L], resolving within ensuing 8 weeks).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, 5 cases were attributed to telithromycin and 3 to azithromycin as single agents, but none to erythromycin or clarithromycin).
- Juricic D, Hrstic I, Radic D, Skegro M, Coric M, Vucelic B, Francetic I. Vanishing bile duct syndrome associated with azithromycin in a 62-year-old man. Basic Clin Pharmacol Toxicol. 2010;106(1):62–5. Erratum in Basic Clin Pharmacol Toxicol 2010; 107(2): 700. [PubMed: 19906050](62 year old man developed rash and fever [Stevens Johnson syndrome] after 3 days of azithromycin therapy which resolved on prednisone, but 2 weeks later jaundice and pruritus appeared [bilirubin 15.0 mg/dL, ALT 1545 U/L, Alk P 545 U/L]; serum enzymes then decreased, but bilirubin levels remained high; liver biopsy showed duct loss and patient developed intractable pruritus and jaundice and underwent liver transplantation 7 months after initial presentation).
- Leitner JM, Graninger W, Thalhammer F. Hepatotoxicity of antibacterials: pathomechanisms and clinical data. Infection. 2010;38:3–11. [PubMed: 20107858](Review; the macrolide antibiotics may cause cholestatic hepatitis at an estimated rate of 3.6 per 100,000).
- Lockwood AM, Cole S, Rabinovich M. Azithromycin-induced liver injury. Am J Health Syst Pharm. 2010;67:810–4. [PubMed: 20479103](69 year old woman developed jaundice and pruritus 4 days after starting azithromycin [bilirubin 2.0 rising to 18.5 mg/dL, ALT 1065 U/L, Alk P 125 U/L], resolving within 2 months of stopping: Case 2).
- Caramaschi P, Mahamid H, Bambara LM, Biasi D. Liver impairment after concomitant administration of bosentan and clarithromycin in systemic sclerosis. Joint Bone Spine. 2010;77:81–2. [PubMed: 20022782](Two cases, 47 and 69 year old women with systemic sclerosis were treated with bosentan without difficulty, but developed ALT elevations [peak 503 and 94 U/L, Alk P 263 and 293 U/L] without jaundice within 5-7 days of starting clarithromycin, which resolved with stopping both or either, attributed to drug-drug interactions).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 31 were due to antibiotics including one due to clarithromycin, but none were attributed solely to azithromycin or erythromycin).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database containing 9036 hepatic adverse drug reactions in children includes 63 cases attributed to azithromycin, 60 to erythromycin and 35 to clarithromycin).
- Das BK. Azithromycin induced hepatocellular toxicity and hepatic encephalopathy in asymptomatic dilated cardiomyopathy. Indian J Pharmacol. 2011;43:736–7. [PMC free article: PMC3229800] [PubMed: 22144789](76 year old man developed nausea and abdominal pain 3 days after starting azithromycin and ranitidine followed by jaundice, itching and confusion [bilirubin 7 mg/dL, ALT 1640 U/L, Alk P 1894 U/L, INR 2.2], resolving rapidly on stopping).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, et al. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including 6 [1.1%] attributed to macrolide antibiotics).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to azithromycin or other macrolide antibiotics).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, one of which was attributed to clarithromycin [acute liver failure], but none were attributed to azithromycin).
- Ferrajolo C, Coloma PM, Verhamme KM, Schuemie MJ, de Bie S, Gini R, Herings R, et al. EU-ADR consortium. Signal detection of potentially drug-induced acute liver injury in children using a multi-country healthcare database network. Drug Saf. 2014;37:99–108. [PubMed: 24446276](Analyses of large spontaneous reporting databases from 3 European countries between 1995 and 2010 identified 125 drugs with at least one exposed case of unexplained acute liver injury in children, 20 of which had a statistically significant association, including clarithromycin [5 cases] and erythromycin [4 cases], but not azithromycin).
- Kaye JA, Castellsague J, Bui CL, Calingaert B, McQuay LJ, Riera-Guardia N, Saltus CW, et al. Risk of acute liver injury associated with the use of moxifloxacin and other oral antimicrobials: a retrospective, population-based cohort study. Pharmacotherapy. 2014;34:336–49. [PMC free article: PMC4260122] [PubMed: 24865821](In a nested case control analysis of a health care network database of persons between 2001 and 2009, 8 selected antibiotics were assessed for association with risk of hospitalization for liver injury, adjusted relative risks being significantly elevated for levofloxacin [3.2], moxifloxacin [2.3], doxycycline [2.5], amoxicillin/clavulanate [2.5] and amoxicillin [2.3], but not for clarithromycin [1.8], telithromycin [1.7] or cefuroxime [0.9]).
- Moy BT, Dojki FK, Scholes JV, Hoffman MG. Azithromycin-induced cholestatic hepatitis. Conn Med. 2015;79:213–5. [PubMed: 26259299](65 year old man developed jaundice 2 weeks after starting a 5 day course of azithromycin [bilirubin 17.2 mg/dL, ALT 63 U/L, Alk P 613 U/L, INR 1.1], resolving clinically over the next several months, but with persistent Alk P elevations).
- Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, Gu J, et al. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369–376.e3. [PMC free article: PMC4321982] [PubMed: 25111234](Among 18 patients with azithromycin induced liver injury enrolled in a US database between 2004 and 2013, the average age was 37 [range 2 to 76] years, 72% were women, latency averaged 17 days, 89% had jaundice, 44% had a cholestatic or mixed and 56% hepatocellular pattern, 1 case was fatal and 1 underwent liver transplant, 29% had chronic liver test abnormalities, and 2 had severe cutaneous reactions).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 323 [36%] were attributed to antibiotics including 29 due to macrolides of which 18 were linked to azithromycin [2%], making it the 7th most frequently implicated medication).
- Koffas A, Murray-Lyon IM, Williams R. Azithromycin-induced cholestatic hepatitis. Oxf Med Case Reports. 2017;2017:omx027. [PMC free article: PMC5451892] [PubMed: 28580159](69 year old man developed fever and jaundice 2 weeks after a 3 day course of azithromycin [bilirubin 12.3 rising to 21.1 mg/dL, ALT 415 U/L, Alk P 145 rising to 768 U/L, INR 0.9], resolving within 2 months with prednisone therapy that was later withdrawn without relapse).
- Ferrajolo C, Verhamme KM, Trifirò G, 't Jong GW, Picelli G, Giaquinto C, Mazzaglia G, et al. Antibiotic-induced liver injury in paediatric outpatients: a case-control study in primary care databases. Drug Saf. 2017;40:305–15. [PMC free article: PMC5362651] [PubMed: 28025733](In a health care database of 429,772 children in Italy and the Netherlands followed between 2008 and 2010, 938 cases of liver injury of uncertain cause were identified, the rate being higher in those with current use of antibiotics [12% vs 3.6%] for an adjusted odds rate ratio [aOR] of 3.2; specific antibiotics most commonly implicated were fluoroquinolones [19.0], cephalosporins [4.5], macrolides [3.5] and penicillins [2.6], and a specific aOR for azithromycin of 2.4).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss, including 2 cases attributed to azithromycin both of whom developed evidence for chronic liver injury suggestive of vanishing bile duct syndrome).
- Han B, Sheng Y, Wang L, Feng H, Hou X, Li Y. Intrahepatic cholestasis of pregnancy or azithromycin-induced intrahepatic cholestasis: A case report. Medicine (Baltimore). 2017;96:e9346. [PMC free article: PMC6392943] [PubMed: 29384912](30 year old woman in 38th week of pregnancy developed pruritus 4 days after starting azithromycin [bilirubin not given, ALT 37 Alk P 240 U/L, bile acids 10 fold elevated] which was thought to be either intrahepatic cholestasis of pregnancy or azithromycin liver injury, with full resolution within 10 days of stopping drug and Caesarian section).
- Ellison CA, Blackwell SB. Acute Hepatocellular Injury Associated With Azithromycin. J Pharm Pract. 2021;34(3):493–6. [PubMed: 31928122](83 year old man with pneumonia treated with multiple antibiotics including azithromycin developed elevations in ALT [1002 U/L], AST [1895 U/L] and LDH [2051 U/L], with minimal increase in Alk P [peak=125 U/L] and bilirubin [peak=2.2 mg/dL], one day later which continued to rise after stopping other drugs until azithromycin was stopped on day 5 with prompt improvement).
- Ramírez E, Urroz M, Rodríguez A, González-Muñoz M, Martín-Vega A, Villán Y, Seco E, et al. Incidence of suspected serious adverse drug reactions in corona virus disease-19 patients detected by a pharmacovigilance program by laboratory signals in a tertiary hospital in Spain: cautionary data. Front Pharmacol. 2020;11:602841. [PMC free article: PMC7744878] [PubMed: 33343374](Among 2682 hospitalized patients with COVID-19, routine laboratory data suggested that severe adverse events were 3-fold more common than among patients without COVID-19 [from 2020 and 2019], higher rates being for hepatic, renal and muscle signals in COVID-19 patients and seen during therapy with many of the drugs used including azithromycin).
- Gyselinck I, Janssens W, Verhamme P. os R. Rationale for azithromycin in COVID-19: an overview of existing evidence. BMJ Open Respir Res. 2021;8:e000806. [PMC free article: PMC7811960] [PubMed: 33441373](Review of the rationale for use of azithromycin in COVID-19 infection including its effects on receptor binding, endosomal acidification, alternations in metabolic and inflammatory pathways, effects on cytokine production and function and on T cells, B cells and neutrophils).
- Dauner DG, Dauner KN. Summary of adverse drug events for hydroxychloroquine, azithromycin, and chloroquine during the COVID-19 pandemic. J Am Pharm Assoc (2003) 2021: S1544-3191(21)00008-X. [PMC free article: PMC7833798] [PubMed: 33546986](Review of the FDA adverse drug reports for January to July 2020 for azithromycin, chloroquine and hydrochloroquine, including rise in reports from 592 before to 2492 after their emergency use authorization for COVID-19 infection, mostly for hydroxychloroquine [596] and azithromycin [184], the second most frequent event being “hepatitis”).
- Melo JRR, Duarte EC, Moraes MV, Fleck K, Silva ASDNE, Arrais PSD. Adverse drug reactions in patients with COVID-19 in Brazil: analysis of spontaneous notifications of the Brazilian pharmacovigilance system. Cad Saude Publica. 2021;37:e00245820. [PubMed: 33503163](In Brazil between March and August 2020, a total of 631 adverse event reports in 402 patients with COVID-19 were received between March and August 2020, of which 56 cases [9%] were hepatic including 28 attributed to hydroxychloroquine [2 fatal], 4 azithromycin [1 fatal], and 2 chloroquine [2 fatal]).
- RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605–12. [PMC free article: PMC7884931] [PubMed: 33545096](Among 2582 hospitalized patients with COVID-19 pneumonia randomized to receive azithromycin [500 mg daily for 10 days] vs 5181 given usual care, the mortality rate in the two groups was identical [22%] and duration of hospitalization similar [10 vs 11 days]; only one serious adverse event was reported due to azithromycin: a self-limited case of pseudomembranous colitis).
- PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–74. [PMC free article: PMC7972318] [PubMed: 33676597](In an adaptive UK based study with a randomized trial of azithromycin [500 mg daily for 3 days] or usual care in patients with suspected COVID-19 who were above the age of 65 years [or above the age of 50 with comorbidities], the rates of recovery by 28 days were similar [80% vs 77%] and there were no deaths and few adverse events that could be related to drug).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Clarithromycin.[LiverTox: Clinical and Researc...]Review Clarithromycin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Adverse events in people taking macrolide antibiotics versus placebo for any indication.[Cochrane Database Syst Rev. 2019]Adverse events in people taking macrolide antibiotics versus placebo for any indication.Hansen MP, Scott AM, McCullough A, Thorning S, Aronson JK, Beller EM, Glasziou PP, Hoffmann TC, Clark J, Del Mar CB. Cochrane Database Syst Rev. 2019 Jan 18; 1(1):CD011825. Epub 2019 Jan 18.
- Review Macrolide antibiotics for cystic fibrosis.[Cochrane Database Syst Rev. 2011]Review Macrolide antibiotics for cystic fibrosis.Southern KW, Barker PM, Solis-Moya A, Patel L. Cochrane Database Syst Rev. 2011 Dec 7; (12):CD002203. Epub 2011 Dec 7.
- Assessment of Long-Term Macrolide Exposure on the Oropharyngeal Microbiome and Macrolide Resistance in Healthy Adults and Consequences for Onward Transmission of Resistance.[Antimicrob Agents Chemother. 2...]Assessment of Long-Term Macrolide Exposure on the Oropharyngeal Microbiome and Macrolide Resistance in Healthy Adults and Consequences for Onward Transmission of Resistance.Burr LD, Taylor SL, Richard A, Schreiber V, Lingman S, Martin M, Papanicolas LE, Choo JM, Rogers GB. Antimicrob Agents Chemother. 2022 Apr 19; 66(4):e0224621. Epub 2022 Mar 16.
- Antibiotic therapy for pelvic inflammatory disease.[Cochrane Database Syst Rev. 2020]Antibiotic therapy for pelvic inflammatory disease.Savaris RF, Fuhrich DG, Maissiat J, Duarte RV, Ross J. Cochrane Database Syst Rev. 2020 Aug 20; 8(8):CD010285. Epub 2020 Aug 20.
- Azithromycin - LiverToxAzithromycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...