NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Asenapine is a second generation (atypical) antipsychotic agent that is taken sublingually and used in the treatment of schizophrenia and manic or mixed episodes associated with bipolar 1 disorder. Asenapine is associated with a low rate of transient and mild serum aminotransferase elevations during therapy but has not been linked to instances of clinically apparent acute liver injury.
Background
Asenapine (a sen' a peen) is a second generation antipsychotic agent which appears to act as a dopamine type 2 (D2) and serotonin (5-HT)-2A receptor antagonist. It is a somewhat unique antipsychotic agent that has a tetracyclic structure similar to that of mirtazapine, and it is administered as a sublingual tablet, being poorly absorbed by the oral route. Several randomized controlled trials have shown that sublingual asenapine improves symptoms of schizophrenia with effects comparable to risperidone and olanzapine. It also has beneficial activity in acute manic and mixed episodes associated with bipolar 1 disorder. Asenapine was approved for use in the United States in 2009 and is available in sublingual tablets of 2.5, 5 and 10 mg generically and under the brand name Saphris. The typical maintenance dose in adults is 2.5 to 10 mg twice daily. Asenapine is also available for treatment of schizophrenia as a transdermal formulation available in doses of 3.8, 5.7 and 7.6 mg per 24 hours under the brand name Secuado. The recommended initial dose of the transdermal patch is 3.8/24 hours, which can be increased to 5.7/24 hours and 7.6 mg/24 hours based upon effect and tolerance. Common side effects of asenapine include dizziness, somnolence, fatigue, nausea, anxiety, restlessness (akathisia) and weight gain. Rare, but potentially severe adverse reactions (mentioned in most antipsychotic and antidepressant product labels) include tardive dyskinesia, major neurologic events, neuroleptic malignant syndrome, orthostatic hypotension, seizures, neutropenia, hypersensitivity reactions, prolongation of the QTc interval, increased cerebrovascular events, and suicidal ideation or behavior. Asenapine also has a boxed warning of increased mortality in elderly patients with dementia-related psychosis.
Hepatotoxicity
Liver test abnormalities occur in 1% to 2.5% of patients receiving asenapine, but similar rates are reported with placebo therapy (0.6% to 1.3%) and with comparator agents. The ALT elevations are usually mild, transient and often resolve even without dose modification or drug discontinuation. There has been a single case report of cholestatic serum enzyme elevations arising 3 to 4 weeks after starting asenapine, resolving within a month of stopping. Thus, asenapine may be a rare cause of mild cholestatic liver injury.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which asenapine might cause serum enzyme elevations or liver injury is not known. Asenapine is metabolized to some extent by the cytochrome P450 system (CYP 2D6 and 3A4), but is an uncommon cause of significant drug-drug interactions with agents that inhibit or induce these microsomal enzymes.
Outcome and Management
The serum aminotransferase elevations that occur with asenapine therapy are usually self-limited and often do not require dose modification or discontinuation. No instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome have been attributed to asenapine. Cross sensitivity to liver related or other hypersensitivity reactions between asenapine and other antipsychotic agents have not been demonstrated.
Drug Class: Antipsychotic Agents, Atypicals
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Asenapine – Saphris®, Secuado®
DRUG CLASS
Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
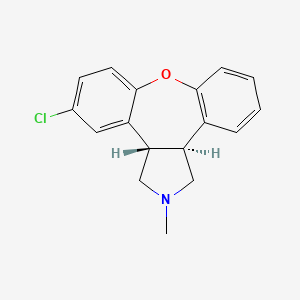
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Asenapine | 65576-45-6 | C17-H16-Cl-N-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 05 June 2023
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Larry D. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 507-26.(Review of hepatotoxicity of psychiatric agents, does not discuss asenapine).
- Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68:1492–500. [PubMed: 17960962](Among 174 patients with acute schizophrenia treated with sublingual asenapine [5 mg] or risperidone [3 mg] or placebo twice daily for 6 weeks, symptom scores improved more with asenapine and risperidone than with placebo, although adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–10. [PubMed: 19321312](Analysis of weight gain in 21 placebo controlled trials [~3300 patients]; average monthly weight gain in pounds was +0.1 with placebo, +0.8 olanzapine, 0.6 risperidone, -0.3 ziprasidone; a 5% increase in weight occurred after one year in 13% of placebo, 39% haloperidol, 20% ziprasidone, 45% risperidone and 60% olanzapine treated subjects; no mention of asenapine).
- McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J. A 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed states. Bipolar Disord. 2009;11:673–86. [PubMed: 19839993](Among 480 adults with an acute manic or mixed episode in a prospective controlled trial for 3 weeks, there was rapid improvement in symptoms with sublingual asenapine [5 mg] and oral olanzapine [3 mg] compared to placebo twice daily; ALT levels increase by 12 U/L on average with asenapine, 33 U/L with olanzapine, but decrease by 7 U/L with placebo, although none of the changes were considered “clinically relevant”).
- McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J. Asenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2010;122(1-2):27–38. [PubMed: 20096936](Among 488 adults with acute mania and bipolar 1 disorder treated with asenapine, olanzapine or placebo for 3 weeks, symptoms score improved more in the asenapine than placebo recipients and “mean changes from baseline in laboratory values…were not clinically significant”).
- Asenapine (Saphris) sublingual tablets for schizophrenia and bipolar disorder. Med Lett Drugs Ther. 2010;52:9–10. [PubMed: 20216523](Concise review of the mechanism of action, pharmacology, efficacy, safety and costs of asenapine as therapy of schizophrenia and bipolar disorder shortly after its approval for use in the US, mentions side effects of akathisia, oral hypoesthesia, somnolence, dizziness and weight gain; no mention of ALT elevations or hepatotoxicity).
- Potkin SG. Asenapine: a clinical overview. J Clin Psychiatry. 2011;72 Suppl 1:14–8. [PubMed: 22217438](Review and overview of the pharmacology, clinical efficacy and safety of sublingual asenapine for treatment of schizophrenia and acute mania mentions occasional hypersensitivity reactions and problems of weight gain with long term therapy; no mention of ALT elevations or hepatotoxicity).
- Asenapine: a less effective, yet more dangerous neuroleptic! Prescrire Int. 2012;21:229–32. [PubMed: 23185842](Short commentary on use of asenapine in bipolar disorders mentions that asenapine has more adverse side effects than other neuroleptics, and these include oral hypoesthesia and hypersensitivity reactions that can be severe).
- Cazorla P, Mackle M, Zhao J, Ha X, Szegedi A. Safety and tolerability of switching to asenapine from other antipsychotic agents: pooled results from two randomized multicenter trials in stable patients with persistent negative symptoms in schizophrenia. Neuropsychiatr Dis Treat. 2012;8:247–57. [PMC free article: PMC3383320] [PubMed: 22745558](Among 949 adults with schizophrenia who were switched from standard antipsychotic agents to asenapine or olanzapine in two 26-week controlled trials, common side effects in the first month included somnolence [10% vs 13%], insomnia [17% vs 12%], and headache [14% vs 10%], while serious adverse events occurred in 12% on asenapine and 6% on olanzapine, although no specifics given or mention made of ALT elevations).
- Schoemaker J, Stet L, Vrijland P, Naber D, Panagides J, Emsley R. Long-term efficacy and safety of asenapine or olanzapine in patients with schizophrenia or schizoaffective disorder: an extension study. Pharmacopsychiatry. 2012;45:196–203. [PubMed: 22454251](Among 440 patients with schizophrenia or schizoaffective disorder who were continued on asenapine or olanzapine after a 52 week controlled trial, adverse events were common, but less frequent than during the initial year of treatment, and there were “no major changes in laboratory variables including … liver enzymes”).
- Potkin SG, Phiri P, Szegedi A, Zhao J, Alphs L, Cazorla P. Long-term effects of asenapine or olanzapine in patients with persistent negative symptoms of schizophrenia: a pooled analysis. Schizophr Res. 2013;150:442–9. [PubMed: 24075603](Among 502 patients with schizophrenia treated with asenapine or olanzapine in two clinical trials for 26 weeks with another 26 week extension study, adverse events were not discussed in detail and no mention made of ALT elevations or hepatotoxicity).
- Drugs for psychiatric disorders. Treat Guidel Med Lett. 2013;11(130):53–64. [PubMed: 23715100](Concise review of safety, efficacy and role of drugs for psychiatric disorders mentions that asenapine is a second generation antipsychotic agent whose adverse side effects include insomnia, somnolence, nausea, vomiting and weight gain; no mention of ALT elevations or hepatotoxicity).
- Kemp DE, Zhao J, Cazorla P, Landbloom RP, Mackle M, Snow-Adami L, Szegedi A. Weight change and metabolic effects of asenapine in patients with schizophrenia and bipolar disorder. J Clin Psychiatry. 2014;75:238–45. [PubMed: 24499969](Among 1748 patients with schizophrenia or bipolar illness treated in 17 clinical trials, post hoc analyses of weight change showed greater gain with asenapine than placebo during the initial 4 week period [1.2 vs 0.4 kg], but less than with olanzapine [0.9 vs 3.0 kg]; no mention of changes of ALT levels with weight gain).
- Musil R, Obermeier M, Russ P, Hamerle M. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14:73–96. [PubMed: 25400109](Extensive systematic review of the literature on the problem of weight gain during therapy with antipsychotic agents mentions that asenapine has been linked to weight gain and increase in body weight of 7% or more occurs in 3.7-39.2% of patients).
- Landbloom RL, Mackle M, Wu X, Kelly L, Snow-Adami L, McIntyre RS, Mathews M, et al. Asenapine: Efficacy and safety of 5 and 10 mg bid in a 3-week, randomized, double-blind, placebo-controlled trial in adults with a manic or mixed episode associated with bipolar I disorder. J Affect Disord. 2016;190:103–10. [PubMed: 26496015](Among 367 patients with a manic or mixed episode associated with bipolar 1 disorder treated with asenapine [5 or 10 mg] or placebo twice daily for 3 weeks, symptoms were somewhat more improved with asenapine than placebo, while adverse events were more frequent, particularly somnolence [20% and 26% vs 4%] and oral hypoesthesia [16% and 29% vs 2%], although “no patients had elevated liver enzymes that met criteria for potential drug-induced liver injury”).
- Findling RL, Landbloom RP, Mackle M, Pallozzi W, Braat S, Hundt C, Wamboldt MZ, et al. Safety and efficacy from an 8 week double-blind trial and a 26 week open-label extension of asenapine in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2015;25:384–96. [PMC free article: PMC4491161] [PubMed: 26091193](Among 306 adolescents with schizophrenia treated with asenapine [2.5 or 5 mg] or placebo twice daily for 8 weeks, some symptom scores improved with the higher dose of asenapine compared to placebo, but adverse events were also more common including akathisia, dizziness, oral hypoesthesia, insomnia and weight gain; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 patients with drug induced liver injury seen over a ten year period at 8 US medical centers, one case was attributed to olanzapine, but none to asenapine or other atypical antipsychotic medications).
- Schultz K, Wang L, Barr A, Vila Rodriguez F. A case of pseudo-Stauffer's syndrome related to asenapine use. Schizophr Res. 2015;169:500–1. [PubMed: 26549631](50 year old man developed abnormal liver tests 24 days after starting asenapine [bilirubin and ALT not given; Alk P 508 U/L, GGT 904 U/L], which persisted despite stopping valproate and then resolved within a month of stopping asenapine).
- Findling RL, Landbloom RL, Mackle M, Wu X, Snow-Adami L, Chang K, Durgam S. Long-term safety of asenapine in pediatric patients diagnosed with bipolar I disorder: A 50-week open-label, flexible-dose trial. Paediatr Drugs. 2016;18:367–78. [PMC free article: PMC5018262] [PubMed: 27461426](Among 321 pediatric patients [ages 10 to 17 years] with bipolar disorder were treated with sublingual asenapine for up to one year, side effects were generally mild but led to early discontinuation in 15% of patients; no mention of ALT elevations or hepatotoxicity).
- Ketter TA, Durgam S, Landbloom R, Mackle M, Wu X, Mathews M. Long-term safety and tolerability of asenapine: A double-blind, uncontrolled, long-term extension trial in adults with an acute manic or mixed episode associated with bipolar I disorder. J Affect Disord. 2017;207:384–92. [PubMed: 27755982](Among 164 adults with bilar disorder enrolled in a 26 week extension study after a placebo controlled 3 week trial of asenapine, adverse events were mostly neuropsychiatric and weight gain; no mention of ALT elevations or hepatotoxicity).
- Szegedi A, Durgam S, Mackle M, Yu SY, Wu X, Mathews M, Landbloom RP. Randomized, double-blind, placebo-controlled trial of asenapine maintenance therapy in adults with an acute manic or mixed episode associated with bipolar I disorder. Am J Psychiatry. 2018;175:71–79. [PubMed: 28946761](Among 253 patients with acute manic or mixed episodes treated with asenapine [10 mg twice daily] for 12-16 weeks and if they responded were then continued therapy or were switched to placebo, recurrence of manic, mixed, or depressive episodes were less frequent with continuing sublingual asenapine than switching to placebo, and adverse event rates were similar; no mention of ALT elevations or hepatotoxicity).
- Citrome L, Walling DP, Zeni CM, Starling BR, Terahara T, Kuriki M, Park AS, et al. Efficacy and safety of HP-3070, an asenapine transdermal system, in patients with schizophrenia: a phase 3, randomized, placebo-controlled study. J Clin Psychiatry. 2020;82:20m13602. [PubMed: 33326711](Among 614 patients with an acute exacerbation of schizophrenia treated with transdermal asenapine [3.8 or 7.6 mg/24 hours] or placebo for 6 weeks, symptom scores improved more with both doses of asenapine compared with placebo and adverse event rates were similar with no serious adverse events; no mention of ALT elevations or hepatotoxicity).
- In brief: An asenapine patch (Secuado) for schizophrenia. Med Lett Drugs Ther. 2021;63(1615):7–8. [PubMed: 33647000](Concise review of the mechanism of action, clinical efficacy, toxicity and cost of transdermal asenapine shortly after its approval as therapy of schizophrenia in the US; no mention of ALT elevations or hepatotoxicity).
- Zeiss R, Hafner S, Schönfeldt-Lecuona C, Connemann BJ, Gahr M. Drug-associated liver injury related to antipsychotics: exploratory analysis of pharmacovigilance data. J Clin Psychopharmacol. 2022;42:440–444. [PubMed: 35730552](Review of the VigiBase data base of individual case safety reports on antipsychotics and liver injury found positive hepatic safety signals for olanzapine and clozapine but none for risperidone, quetiapine, ziprasidone, asenapine, aripiprazole, brexpiprazole, and cariprazine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic.[Int J Clin Pract. 2009]Review Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic.Citrome L. Int J Clin Pract. 2009 Dec; 63(12):1762-84. Epub 2009 Oct 14.
- Post hoc analyses of asenapine treatment in pediatric patients with bipolar I disorder: efficacy related to mixed or manic episode, stage of illness, and body weight.[Neuropsychiatr Dis Treat. 2018]Post hoc analyses of asenapine treatment in pediatric patients with bipolar I disorder: efficacy related to mixed or manic episode, stage of illness, and body weight.Findling RL, Earley W, Suppes T, Patel M, Wu X, Chang CT, McIntyre RS. Neuropsychiatr Dis Treat. 2018; 14:1941-1952. Epub 2018 Aug 2.
- Review Asenapine: a review of acute and extension phase data in bipolar disorder.[CNS Neurosci Ther. 2011]Review Asenapine: a review of acute and extension phase data in bipolar disorder.McIntyre RS. CNS Neurosci Ther. 2011 Dec; 17(6):645-8. Epub 2010 Oct 15.
- Review Asenapine for bipolar disorder.[Neuropsychiatr Dis Treat. 2015]Review Asenapine for bipolar disorder.Scheidemantel T, Korobkova I, Rej S, Sajatovic M. Neuropsychiatr Dis Treat. 2015; 11:3007-17. Epub 2015 Dec 4.
- Review Cariprazine.[LiverTox: Clinical and Researc...]Review Cariprazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Asenapine - LiverToxAsenapine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...