NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Amphotericin B is an antifungal agent with a broad spectrum of activity against many fungal species. Amphotericin B commonly causes mild to moderate serum aminotransferase elevations and can cause hyperbilirubinemia, but acute, clinically apparent drug induced liver injury from amphotericin B therapy is exceedingly rare.
Background
Amphotericin (am" foe ter' i sin) B is a polyene macrolide antibiotic that acts by binding to sterols in the plasma membranes of fungi causing the cells to leak, eventually leading to fungal cell death. Amphotericin B is indicated for the treatment of a several fungal and other infections including leishmaniasis, invasive aspergillosis, blastomycosis, candidiasis, coccidiomycosis, cryptococcal meningitis, cryptococcosis, histoplasmosis, mucormycosis, sporotrichosis, and others. Amphotericin B was approved by the FDA in 1971 and is currently widely used in the treatment of serious fungal infections. Amphotericin B is insoluble in water and is formulated for intravenous use by complexing it with lipotrophic molecules such as deoxycholate, liposomes or lipid complexes. Amphotericin is available in multiple forms and concentrations generically and under the brand names Amphocin and Fungizone (deoxycholate), Ambisome (liposome), Abelcet (lipid complex), and Amphotec (cholesteryl sulfate complex). The recommended dose varies by drug form and by disease entity; amphotericin B is given intravenously and the usually dosage is up 0.5 to 1.0 mg/kg daily. Many fungal infections require prolonged therapy (for 1 month to as many as 9 months). Common side effects include fever and chills, nausea, weight loss, headache, malaise, azotemia, hypokalemia, anemia, renal tubular acidosis and phlebitis at peripheral vein infusion sites.
Hepatotoxicity
Mild and transient elevations in liver enzymes occur in up to 20% of patients receiving amphotericin. Clinically apparent hepatotoxicity is rare, but several convincing cases have been published. The liver injury arises as early as 4 to 14 days after starting therapy, typically with a hepatocellular or mixed pattern of enzyme elevation. Most patients have no symptoms or jaundice. Recovery occurs promptly upon stopping therapy. In addition, isolated but dramatic instances of hyperbilirubinemia arising within days of starting amphotericin have been reported with elevations largely in the direct (conjugated) bilirubin fraction. These patients become visually jaundiced but have no constitutional symptoms, minimal if any elevations in serum ALT or alkaline phosphatase levels, and no evidence of frank hepatic injury. Finally, rare instances of acute cholestatic hepatitis with jaundice have been reported in patients receiving amphotericin, but these patients have generally been critically ill and exposed to multiple potentially hepatotoxic agents, so that the attribution to amphotericin has been weak.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The cause of serum aminotransferase elevations during amphotericin B therapy is unknown, but may be a direct hepatotoxicity from the polyene based upon its effects on cell membranes. There is little evidence of hypersensitivity, although the injury is idiosyncratic and usually recurs with reexposure and, thus, may be due to a genetic predisposition based upon the metabolism of amphotericin B which is largely hepatic. The hyperbilirubinemia of amphotericin therapy is likely due to inhibition of bilirubin transport mechanisms and is likely to occur in patients with genetic variations in the major hepatic bilirubin transporter (MRP2).
Outcome and Management
The severity of the liver injury due to amphotericin is usually mild, most patients being asymptomatic and anicteric. Recovery is rapid with stopping amphotericin but can recur with rechallenge. No instances of acute liver failure or chronic liver injury have been convincingly linked to amphotericin therapy. There appears to be no cross sensitivity of the hepatotoxicity of amphotericin to other antifungal agents.
Drug Class: Antifungal Agents
CASE REPORT
Case 1. Serum aminotransferase elevations during amphotericin B therapy.
[Modified from: Gill J, Sprenger H, Ralph E, Sharpe M. Hepatotoxicity possibly caused by amphotericin B. Ann Pharmacother 1999; 33: 683-5. PubMed Citation]
A 26 year old man developed elevations in serum aminotransferase levels 3 days after starting intravenous amphotericin B for pulmonary blastomycosis. He had received oral itraconazole for 7 days without much improvement before starting amphotericin B. When serum enzymes continued to rise (Table), amphotericin B was stopped while itraconazole was maintained. He was asymptomatic of liver disease, and tests for viral hepatitis and autoantibodies were negative. An abdominal ultrasound showed no evidence of biliary obstruction. A percutaneous liver biopsy showed mild focal fatty change and little evidence of inflammation or hepatocellular necrosis. Stains for fungal elements were negative. Within 4 days of stopping amphotericin B, serum enzymes had returned to baseline.
Key Points
| Medication: | Amphotericin B (50 mg iv daily; total dose: 225 mg) |
|---|---|
| Pattern: | Hepatocellular (R=8.2) |
| Severity: | 1+ (no jaundice) |
| Latency: | 3 days |
| Recovery: | 4 days |
| Other medications: | Itraconazole, lorazepam, ranitidine, docusate sodium, propofol, subcutaneous heparin |
Laboratory Values
Comment
The evidence that amphotericin was the cause of the liver test abnormalities was the timing of onset within a few days of starting amphotericin B, and the timing of recovery which was immediate upon stopping. The injury was mild and the decision to stop treatment was based entirely on the fact that the serum ALT level had risen to more than 10 times the upper limit of the normal range. While itraconazole was another possible cause of the abnormalities, the serum enzymes decreased immediately upon stopping amphotericin despite continuing itraconazole.
Case 2. Cholestatic serum enzyme elevations induced by Amphotericin B.
[Modified from: Mohan U, Bush A. Amphotericin B-induced hepatorenal failure in cystic fibrosis. Ped Pulmonol 2002; 33: 497-500. PubMed Citation]
A nine year old girl with cystic fibrosis developed nausea and vomiting on the third day of amphotericin B therapy for suspected pulmonary aspergillosis. Her symptoms worsened and the amphotericin was switched to a liposomal formulation. Serum enzymes, which had been normal, were found to be elevated by day 8 of therapy. Because of severe vomiting, dehydration and hematemesis, all antibiotic and antifungal therapy was stopped. There were moderate elevations in ALT and alkaline phosphatase levels, but serum bilirubin remained normal. All abnormalities resolved within 7 days of stopping amphotericin.
Key Points
| Medication: | Amphotericin B (250 µg/kg/day, increased by 250 µg/kg/day; maximum 1 mg/kg/day; total dose: 168 mg) |
|---|---|
| Pattern: | Cholestatic (R=0.9) |
| Severity: | 1+ |
| Latency: | 8 days |
| Recovery: | 7 days |
| Other medications: | Nebulized colistin, amoxicillin-clavulanic acid, multivitamins, pancreatin, ceftazidime, gentamicin |
Laboratory Values
Comment
While the authors described this case as demonstrating hepatorenal failure, most evidence suggests that the hepatic injury was mild. There was no jaundice and laboratory tests returned to baseline rapidly with the patient’s clinical improvement. The elevations in prothrombin time may have been due to the antibiotic therapy and the nutritional status of the patient, rather than hepatic failure. This case demonstrates the difficulty of attributing hepatic enzyme elevations and liver damage to a medication in a critically ill patient with multiple medical problems who is receiving several potent medications, several of which can also cause hepatic injury (clavulanic acid, ceftazidime).
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Amphotericin B – Generic, Amphocin®, Fungizone®
DRUG CLASS
Antifungal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
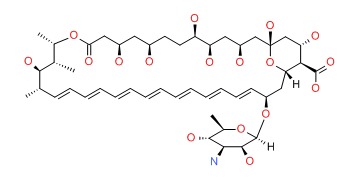
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Amphotericin B | 1397-89-3 | C47-H73-N-O17 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 08 April 2016
- Zimmerman HJ. Antifungal agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 609-11.(Expert review of hepatotoxicity of antifungal agents published in 1999; “Amphotericin B has rarely been incriminated in hepatic injury”; “The rarity of cases despite widespread use of the drug demonstrates its minimal hepatotoxic threat”).
- Moseley RH. Antifungal agents. Antibacterial and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 470-3.(Review of hepatotoxicity of antifungal agents mentions that amphotericin B rarely causes clinically apparent liver injury but several case reports have been published).
- Bennett JE. Antimicrobial agents: antifungal agents. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1571-92. (Textbook of pharmacology and therapeutics.: amphotericin B is insoluble in water and is formulated for iv use by complexing with bile salts or lipids; binds ergosterol in fungal membrane and increases permeability).
- Carnecchia B, Kurtzke J. Fatal toxic reaction to amphotericin B in cryptococcal meningo-encephalitis. Ann Intern Med 1960; 53: 1027-36. [PubMed: 13690877](32 year old man with cryptococcal meningitis and complicated course developed nausea and low grade fever with renal insufficiency and severe phlebitis with each of 4 courses of iv amphotericin [60 to 80 mg/day] developing high fever, large tender liver and pneumonia [bilirubin 1.4 mg/dL, Alk P normal], autopsy showing centrolobular fat, but no inflammation and “some degree of hepatic failure”).
- Miller M. Reversible hepatotoxicity related to amphotericin B. Can Med Assoc J 1984; 131: 1245-7. [PMC free article: PMC1483710] [PubMed: 6594184](51 year old man with acute leukemia developed abnormal ALT [950 U/L], Alk P [150 U/L] and bilirubin [1.6 mg/dL] levels 18 days after starting amphotericin B for pulmonary aspergillosis; asymptomatic and improved on stopping with rapid increase in ALT [100→300 U/L] after 2 day rechallenge).
- Abajo FJ, Carcas AJ. Amphotericin B hepatotoxicity. Br Med J 1986; 293: 1243. Not in PubMed.(29 year old man with HIV infection developed rising Alk P [1000 U/L] and GGT [133 U/L, normal <65] after 10 days of amphotericin B, with improvement on lowering dose; no symptoms and no mention of ALT).
- Stamm AM, Diasio RB, Dismukes WE, Cloud GA, Bowles CA, Karam GH, Espinel-Ingroff A. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am J Med 1987; 83: 236-42. [PubMed: 3303926](Controlled trial of 4 vs 6 weeks of amphotericin B and flucytosine in 194 patients with cryptococcal meningitis; elevated liver enzymes arose during first 4 weeks in 13 [6.2%] patients, and one patient developed signs and symptoms of hepatitis, dying of acute liver failure 1 month later; authors attributed liver injury to flucytosine).
- Ritchie D. Comment: consideration of amphotericin B hepatotoxicity. DCIP 1991; 25: 559-60. [PubMed: 2068844](Author agrees that the case described by Gradon [1991] was likely due to ketoconazole, but stresses that amphotericin B can also cause liver injury).
- Campo C, Antón E, Morata C, Lacruz J. [Amphotericin B associated with severe liver toxicity]. Rev Clin Esp 1999; 199: 49. [PubMed: 10089782](36 year old man with HIV infection and esophageal candidiasis developed nausea and confusion during high dose amphotericin B infusions, with ALT rising within 24 hours to 3145 U/L, bilirubin 1.1 mg/dL, LDH 3,223 U/L, rapid recovery within days).
- Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med 1999; 340: 764-71. [PubMed: 10072411](Trial of two forms of amphotericin in 687 patients with fever and neutropenia, similar efficacy; side effects were less with liposomal forms; ALT or AST >5 x ULN in 17.8% vs 20.3% with standard amphotericin).
- Cook G, Franklin IM. Adverse drug reactions associated with the administration of amphotericin B lipid complex (Abelcet). Bone Marrow Transplant 1999; 23: 1325-6. [PubMed: 10414925](Among 32 patients treated with amphotericin lipid complex, only 2 developed liver test elevations; neither required discontinuation).
- Gill J, Sprenger H, Ralph E, Sharpe M. Hepatotoxicity possibly caused by amphotericin B. Ann Pharmacother 1999; 33: 683-5. [PubMed: 10410179](26 year old man with blastomycosis developed elevations in ALT [518 U/L], Alk P [205 U/L] but not bilirubin [0.8 mg/dL] after 10 days of amphotericin B; liver biopsy showed mild fatty change only; enzyme elevations fell to normal within a few days of stopping amphotericin despite continuing itraconazole: Case 1).
- Persat F, Schwartzbrod PE, Troncy J, Timour Q, Maul A, Piens A, Picot S. Abnormalities in liver enzymes during simultaneous therapy with itraconazole and amphotericin B in leukaemic patients. J Antimicrob Chemother 2000; 45: 928-9. [PubMed: 10837458](Experience in treating 20 patients with itraconazole [12 also on amphotericin] for 44-495 days, ALT elevations in 11 of 12 who received combination therapy, levels 2-10 times ULNl, all resolving, some with stopping amphotericin alone).
- Fleming RV, Kantarjian HM, Husni R, Rolston K, Lim J, Raad I, Pierce S, et al. Comparison of amphotericin B lipid complex (ABLC) vs. ambisome in the treatment of suspected or documented fungal infections in patients with leukemia. Leuk Lymphoma 2001; 40: 511-20. [PubMed: 11426524](Comparison of two formulations of amphotericin B in 75 patients with leukemia and suspected invasive fungal infection; elevations in serum bilirubin above 1.5 mg/dL occurred in 24% but not clinically important; no cases of acute liver injury).
- Ellis M, Shamoon A, Gorka W, Zwaan F, al-Ramadi B. Severe hepatic injury associated with lipid formulations of amphotericin B. Clin Infect Dis 2001; 32: E87-9. [PubMed: 11229863](14 year old girl with leukemia developed abdominal pain and jaundice 21 days after completing a 10 day course of amphotericin [bilirubin 12.7 mg/dL, ALT 168 U/L, Alk P 2679 U/L] and dilation of biliary tract on ultrasound, resolving over several months; not likely due to amphotericin).
- Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, Powderly WG, et al.; U.S. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med 2002; 137: 105-9. [PubMed: 12118965](Randomized trial in 81 patients with AIDS and histoplasmosis; efficacy better [88% vs 64%] and both nephrotoxicity [9% vs 37%] and liver test elevations were less frequent with the liposomal formulation vs standard forms of amphotericin [27% vs 45%]).
- Inselmann G, Inselmann U, Heidemann. Amphotericin B and liver function. Eur J Intern Med 2002; 13: 288-92. [PubMed: 12144907](Review of mechanism of action and hepatic metabolism of amphotericin, focusing on CYP 450 effects which tend to be delayed).
- Mohan U, Bush A. Amphotericin B-induced hepatorenal failure in cystic fibrosis. Ped Pulmonol 2002; 33: 497-500. [PubMed: 12001285](9 year old girl with cystic fibrosis became critically ill while on amphotericin B [bilirubin 0.6 mg/dL, ALT 364 U/L, Alk P 1254 or ~3 times baseline, prothrombin time 27.5 sec], with rapid reversal on stopping amphotericin B, but with other possible causes of liver injury present: Case 2).
- Bowden R, Chandrasekar P, White MH, Li X, Pietrelli L, Gurwith M, van Burik JA, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis 2002; 35: 359-66. [PubMed: 12145716](Controlled trial in 174 patients with aspergillosis found similar efficacy, but less nephrotoxicity [25% vs 49%] and bilirubin elevations [4.5% vs 10.5% ], with colloidal dispersion formulation compared to standard forms of amphotericin).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 6 were attributed to ketoconazole and 1 to itraconazole, but none to amphotericin).
- Fischer MA, Winkelmayer WC, Rubin RH, Avorn J. The hepatotoxicity of antifungal medications in bone marrow transplant recipients. Clin Infect Dis 2005; 41: 301-7. [PubMed: 16007524](Among 438 patients undergoing bone marrow transplantation, 123 developed ALT or AST above 3 times ULN; factors associated with significant increases were liposomal amphotericin [Odds Ratio=3.8] and fluconazole [2.6], but not standard amphotericin [2.0]).
- Wingard J, Leather H. Hepatotoxicity associated with antifungal therapy after bone marrow transplantation. Clin Infect Dis 2005; 41: 308-10. [PubMed: 16007525](Editorial in response to the article by Fisher [2005] discusses the difficulties of detection, diagnosis, attribution and management of liver test abnormalities after bone marrow transplantation).
- Song J, Deresinski S. Hepatotoxicity of antifungal agents. Curr Opin Investig Drugs 2005; 6: 170-7. [PubMed: 15751740](Extensive review of hepatotoxicity from antifungals; ALT elevations occur in 3-19% of patients receiving various formulations of amphotericin: liposomal, lipid, colloid and standard; individual case report of more serious hepatotoxicity).
- Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl 2006; 12: 850-8. [PubMed: 16628697](Metaanalysis found 6 studies with total of 698 patients comparing fluconazole, itraconazole or amphotericin vs placebo for prevention of fungal infections after liver transplantation; side effects were more with prophylaxis but liver toxicity was not discussed).
- Girois SB, Chapuis F, Decullier E, Revol BG. Adverse effects of antifungal therapies in invasive fungal infections: review and meta-analysis. Eur J Clin Microbiol Infect Dis 2006; 25: 138-49. [PubMed: 16622909](Systematic review of adverse effects of antifungal therapy in 54 studies with 9228 patients; hepatotoxicity reported in 14.1-18.6% on amphotericin, 1.9% on fluconazole and 31.6% on itraconazole; however, great variation in definitions and intensity of monitoring).
- Olin JL, Spooner LM. Amphotericin B-associated hyperbilirubinemia: case report and review of the literature. Pharmacotherapy 2006; 26: 1011-7. [PubMed: 16878370](53 year old with lung cancer developed marked increases in direct and total bilirubin [5.5 and 6.1 mg/dL] within 2 days of starting amphotericin with minimal increase in ALT or Alk P, resolving with stopping and recurring with rechallenge using a different formulation).
- Chamilos G, Luna M, Lewis RE, Chemaly R, Raad II, Kontoyiannis DP. Effects of liposomal amphotericin B versus an amphotericin B lipid complex on liver histopathology in patients with hematologic malignancies and invasive fungal infections: a retrospective, nonrandomized autopsy study. Clin Ther 2007; 29: 1980-6. [PubMed: 18035197](Among 64 patients undergoing autopsy after death from cancer and fungal infections, 92% had abnormal liver tests, but histological features of hepatotoxicity were infrequent and mild, consisting of focal fatty change and necrosis).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 8 were attributed to antifungal agents, including 4 to terbinafine, 2 to fluconazole, 1 each to ketaconazole and itraconazole, but none to linked to amphotericin therapy).
- Akyol Erikci A, Ozyurt M, Terekeci H, Ozturk A, Karabudak O, Oncu K. Oesophageal aspergillosis in a case of acute lymphoblastic leukaemia successfully treated with caspofungin alone due to liposomal amphotericin B induced severe hepatotoxicity. Mycoses 2009; 52: 84-6. (18 year old man with acute lymphoblastic leukemia, treated with liposomal amphotericin B for esophageal aspergillosis, developed jaundice [bilirubin 25.1 mg/dL, ALT 914 U/L, Alk P 219 U/L], which improved upon stopping). [PubMed: 18498301]
- Antifungal drugs. Treat Guidel Med Lett 2009; 7: 95-102. [PubMed: 19940816](Concise summary of therapy of fungal infections with recommendations on agents, dosage and duration of treatment and safety; mentions that nephrotoxicity is the most common dose limiting side effect of amphotericin and that liver toxicity has occurred rarely with the lipid formulations).
- Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother 2010; 54: 2409-19. [PMC free article: PMC2876415] [PubMed: 20308378](Systematic review of 39 controlled trials in more than 8000 patients, found liver enzyme elevations in 14.5% of patients on amphotericin, but only 0.2-1.2% stopped therapy for this reason [pooled estimates]).
- Patel GP, Crank CW, Leikin JB. An evaluation of hepatotoxicity and nephrotoxicity of liposomal amphotericin B (L-AMB). J Med Toxicol 2011; 7: 12-5. [PMC free article: PMC3614101] [PubMed: 21057910](Among 75 patients treated with liposomal amphotericin B, 15 [21%] developed ALT or AST elevations >3 times ULN or bilirubin >1.5 mg/dL; but no details given).
- Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013; 73: 919-34. [PubMed: 23729001](Extensive review of the relative safety of newer formulations of amphotericin, lipid-associated preparations having less renal toxicity and fewer infusion reactions than standard deoxycholate formulations; hepatotoxicity is not discussed).
- Michot JM, Gubavu C, Fourn E, Maigne G, Teicher E, Angoulvant A, Blanche S, et al. Very prolonged liposomal amphotericin B use leading to a lysosomal storage disease. Int J Antimicrob Agents 2014; 43: 566-9. [PubMed: 24787480](28 year old man with chronic granulomatous disease developed hepatosplenomegaly having been treated with liposomal amphotericin for 8 years [bilirubin 0.9 mg/dL, ALT 26 U/L, Alk P 698 U/L], liver biopsy showing nodular regenerative hyperplasia and sea-blue foamy macrophages [also found in the bone marrow], suggestive of lysosomal accumulation of lipid from the amphotericin liposomes).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, 6 were attributed to antifungal agents but none to amphotericin).
- Giannella M, Ercolani G, Cristini F, Morelli M, Bartoletti M, Bertuzzo V, Tedeschi S, et al. High-dose weekly liposomal amphotericin b antifungal prophylaxis in patients undergoing liver transplantation: a prospective phase II trial. Transplantation 2015; 99: 848-54. [PubMed: 25531982](Among 76 liver transplant recipients treated with weekly infusions of liposomal amphotericin B for at least 2 weeks, none were reported as developing worsening liver disease or clinically apparent liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 14 were attributed to antifungal agents, but none to amphotericin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.[Drugs. 2009]Review Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Moen MD, Lyseng-Williamson KA, Scott LJ. Drugs. 2009; 69(3):361-92.
- Review Antifungal therapy in infants and children with proven, probable or suspected invasive fungal infections.[Cochrane Database Syst Rev. 2010]Review Antifungal therapy in infants and children with proven, probable or suspected invasive fungal infections.Blyth CC, Hale K, Palasanthiran P, O'Brien T, Bennett MH. Cochrane Database Syst Rev. 2010 Feb 17; 2010(2):CD006343. Epub 2010 Feb 17.
- Review Amphotericin B formulations: a comparative review of efficacy and toxicity.[Drugs. 2013]Review Amphotericin B formulations: a comparative review of efficacy and toxicity.Hamill RJ. Drugs. 2013 Jun; 73(9):919-34.
- A randomized comparison of fluconazole with amphotericin B as empiric anti-fungal agents in cancer patients with prolonged fever and neutropenia.[Am J Med. 1998]A randomized comparison of fluconazole with amphotericin B as empiric anti-fungal agents in cancer patients with prolonged fever and neutropenia.Malik IA, Moid I, Aziz Z, Khan S, Suleman M. Am J Med. 1998 Dec; 105(6):478-83.
- Fluconazole vs low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group.[Bone Marrow Transplant. 2000]Fluconazole vs low-dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group.Wolff SN, Fay J, Stevens D, Herzig RH, Pohlman B, Bolwell B, Lynch J, Ericson S, Freytes CO, LeMaistre F, et al. Bone Marrow Transplant. 2000 Apr; 25(8):853-9.
- Amphotericin B - LiverToxAmphotericin B - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...