NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The amide local anesthetics including lidocaine, bupivacaine and ropivacaine are commonly used for pain control during minor surgery or invasive procedures such as biopsies, small excisions or dental work. These local anesthetics have not been linked to serum enzyme elevations, but when given as constant infusions or repeated injections have been occasionally mentioned as possible causes of clinically apparent liver injury.
Background
Lidocaine (lye' do kane), bupivacaine (bue piv' a kane) and ropivacaine (roe piv' a kane) are commonly used local anesthetics that are chemically referred to as aminoethylamides or amide local anesthetics. Their mechanism of anesthetic action is believed to be based upon inhibition of voltage-gated sodium channels, which results in membrane stabilization and slowing of membrane depolarization and repolarization. Local anesthetics have been shown to be effective and are used widely in preventing or reducing pain from minor surgery, incisions, biopsies, dental and obstetrical procedures and pain from wounds. They are usually given as a single injection locally into a lesion or the area of incision, but can be given as infusions for hours or for several days by epidural or wound-based catheters. Local anesthetic infusions can also be used for postoperative pain management. They have excellent tolerance and safety. Local anesthetics have variable durations of action, and short acting versions are often given with epinephrine, which decreases the rate of absorption limiting systemic exposure and prolonging the duration of action. Lidocaine was approved for use as a local anesthetic in the 1948 originally under the commercial name Xylocaine, ropivacaine in 1990 (Naropin), and bupivacaine in 1990 (Marcaine). Other less commonly used amide anesthetics include articaine (Septocaine: 2000), mepivacaine (Carbocaine: 1960), and prilocaine (Citanest: 1965). A liposomal and longer acting preparation of bupivacaine was approved for use in controlling postsurgical pain in 2011 (Exparel). Side effects of the local anesthetics are usually dose related and occur mostly as a result of systemic administration or exposure. They include neurological symptoms such as drowsiness, tinnitus, dizziness and twitching as well as gastrointestinal effects such as nausea, vomiting and constipation. Cardiovascular depression can also occur and ventricular arrhythmias, especially with higher doses.
Hepatotoxicity
In clinical trials with the amide local anesthetics, serum aminotransferase and alkaline phosphatase elevations were rarely reported and were generally no more common than with placebo or comparator injections. Despite widespread use for many decades, the local amide anesthetics, when given as single or limited injections or infusions, have not been convincingly linked to instances of clinically apparent liver injury, and liver toxicity is not mentioned in product labels. Nevertheless, there have been several case series of jaundice arising a few days to as long as a month after use of bupivacaine given over several days as a constant infusion or as repeated injections into a localized area of pain. Some reports mentioned concurrent administration of an antibiotic such as cefazolin during the perioperative period, which might have accounted for the liver injury. However, in other reports, only bupivacaine was given. The liver injury was mixed or cholestatic and mild-to-moderate in severity, resolving within 1 to 2 months of onset without residual evidence of liver injury. In many instances, there was an accompanying low grade fever, rash or eosinophilia, suggesting a hypersensitivity reaction.
Likelihood score (lidocaine, articaine, mepivacaine, prilocaine, ropivacaine): E (unlikely causes of clinically apparent liver injury).
Likelihood score (bupivacaine): C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which bupivacaine might cause liver injury is unknown, but is most likely due to an idiosyncratic hypersensitivity reaction. The amide local anesthetics are metabolized locally and do not affect the activity of cytochrome P450 enzymes.
Management
The liver injury linked to exposures to amide anesthetics has largely been limited to use of bupivacaine given by infusion or multiple injections over a period of 1 to 3 days. The injury was invariably self-limited, and no instance of chronic liver injury, vanishing bile duct syndrome or acute liver failure has been linked to bupivacaine or other local anesthetics. There have been no reports of recurrence after reexposure to bupivacaine, but in several instances patients with jaundice from bupivacaine infusions have later tolerated injections or infusions with lidocaine.
Drug Class: Anesthetics, Local
CASE REPORT
Case 1. Immunoallergic hepatitis arising 3 weeks after a 2 day course of bupivacaine infusions for postoperative pain control.
[Modified from Case 2 of: Chintamaneni P, Stevenson HL, Malik SM. Bupivacaine drug-induced liver injury: a case series and brief review of the literature. J Clin Anesth 2016; 32: 137-41. PubMed Citation]
A 51 year old man developed fever, dark urine, pruritus and jaundice 3 weeks after an inguinal herniorrhaphy, during which he received bupivacaine injections followed by constant infusions into the area of the incision for two days postoperatively. He also received a single dose of cefazolin at the time of surgery and had taken simvastatin for hyperlipidemia for several years. He recovered from the surgery without complications until several weeks later when symptoms of fever and jaundice arose. He had no previous history of liver disease or risk factors for viral hepatitis. He drank an average of 10 beers weekly. On presentation, he was jaundiced but had no stigmata of chronic liver disease. Laboratory results showed elevations in liver tests, and serum bilirubin subsequently peaked at 9.5 mg/dL, ALT 491 U/L, AST 262 U/L and alkaline phosphatase 378 U/L, yielding an R ratio of ~4.3. The eosinophil count was 9%. He tested negative for markers of acute hepatitis A, B and C and for herpes simplex and Epstein-Barr virus infection. Autoantibodies were not detected. Imaging of the liver by ultrasound and CT showed no evidence of biliary obstruction or hepatic abnormalities. A liver biopsy showed a “mixed” cholestatic and hepatocellular pattern and occasional eosinophils. He recovered rapidly and all liver tests were normal 4 weeks later.
Key Points
| Medication: | Bupivacaine |
| Pattern: | Mixed (R=4.3) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 3 weeks |
| Recovery: | Complete, 1 month after onset |
| Other medications: | Cefazolin, simvastatin |
Comment
Bupivacaine when given by infusion over several days has been linked to at least 10 published cases of acute liver injury, generally arising 12 to 21 days after initiation of the local anesthetic. The clinical pattern of injury has been consistently marked by a mixed or cholestatic hepatitis with immunoallergic features (fever, rash, eosinophilia) and self-limited course, all liver tests returning to normal within 30 to 60 days of onset. The cases have been mild-to-moderate in severity. Strikingly, all instances have been described as a part of case series from a single institution. In the current case, the patient was also exposed to cefazolin, which has also been linked to a delayed cholestatic hepatitis. However, in other instances of liver injury after bupivacaine, no other drugs linked to liver injury were being taken. Other amide local anesthetics have not been implicated in this type of liver injury, but they are rarely given over a period of days and it is not clear whether the association with bupivacaine is specific or whether this type of reaction can occur with the other local anesthetics. In one case series, several patients were later treated with lidocaine for pain control without recurrence of the liver injury. Bupivacaine is similar in structure to the other amide anesthetics and is metabolized locally in many tissues. The cause of the hypersensitivity reaction with liver injury and jaundice remains unclear as does its frequency with different regimens of bupivacaine.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bupivacaine – Generic, Marcaine®, Exparel®
Lidocaine – Generic, Xylocaine®
Ropivacaine – Generic, Naropin®
DRUG CLASS
Anesthetics, Local
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Lidocaine | 137-58-6 | C14-H22-N2-O |
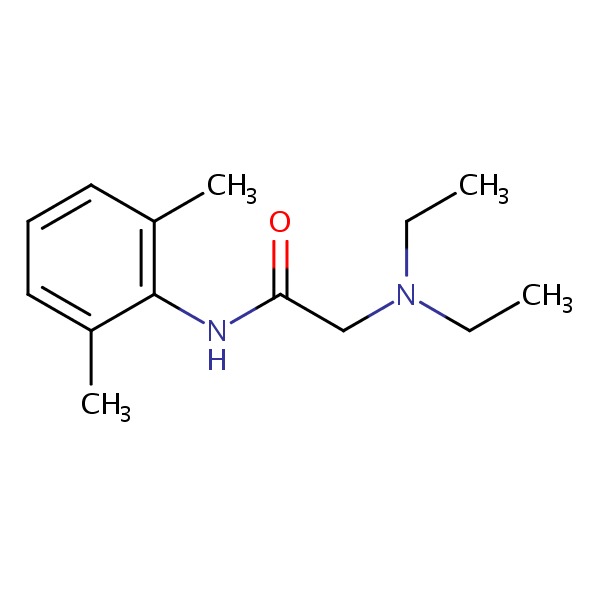 |
| Articaine | 23964-58-1 | C13-H20-N2-O3-S |
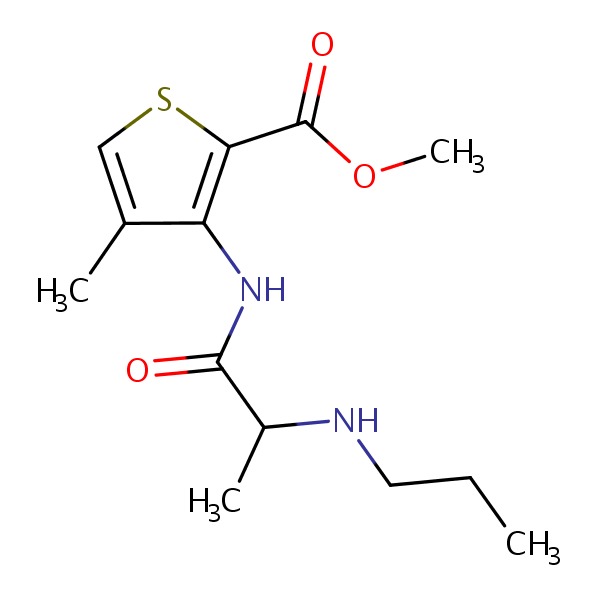 |
| Bupivacaine | 38396-39-3 | C18-H28-N2-O |
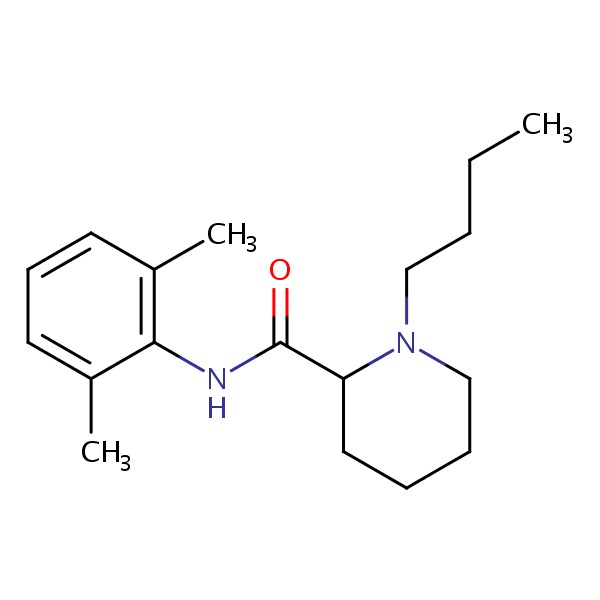 |
| Mepivacaine | 96-88-8 | C15-H22-N2-O |
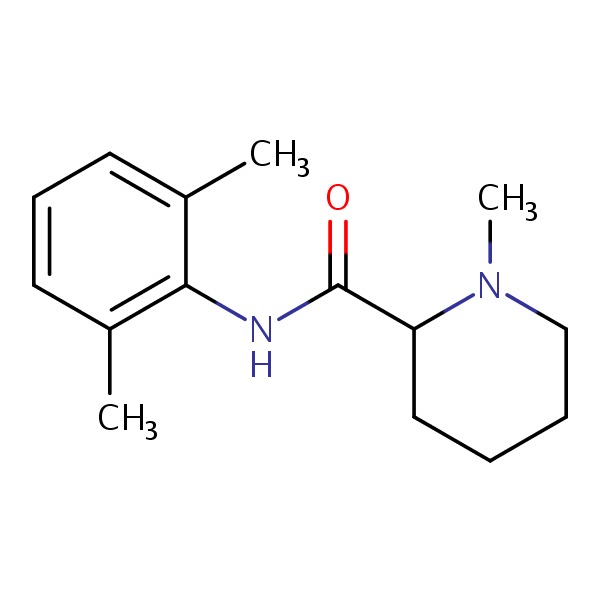 |
| Prilocaine | 721-50-6 | C15-H22-N2-O |
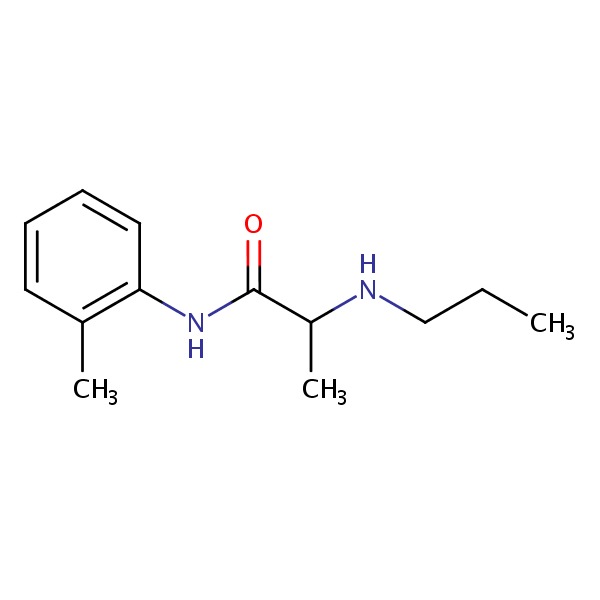 |
| Ropivacaine | 84057-95-4 | C17-H26-N2-O |
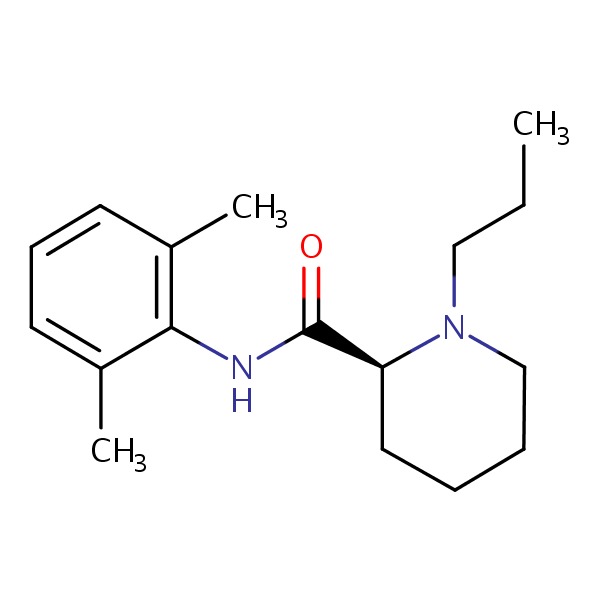 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 July 2017
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999; does not discuss the local anesthetics).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Review of hepatotoxicity published in 2013; does not discuss local anesthetics).
- Catterall WA, Mackie K. Local anesthetics. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 405-20.(Textbook of pharmacology and therapeutics).
- Billström R, Blomberg HM. Cholestasis after interpleural bupivacaine for chronic upper limb pain. Anesth Analg 1993; 76: 1158-9. [PubMed: 8484525](Among 10 patients given repeated infusions of bupivacaine in intrapleural space for chronic arm pain, 3 women, ages 32-55 years, developed liver test abnormalities 2 days to 2 weeks later [bilirubin 0.6- 4.1 mg/dL, ALT 70-495 U/L, Alk P 330-1122 U/L], resolving within 1 month; none received antibiotics).
- Uematsu H, Hiei K, Kawasaki H. [Unknown fever and abnormal liver functions after repeated epidural blocks with lidocaine for management of herpes zoster pain]. Masui 1994; 43: 405-8. Japanese. [PubMed: 8182888](A 67 year old woman received thoracic epidural nerve blocks with lidocaine for 2 weeks when she developed evidence of acute liver injury [described by Chinatmaneni 2016; no further information given]).
- Ropivacaine--a new local anesthetic. Med Lett Drugs Ther 1997; 39 (1007): 80. [PubMed: 9269750](A concise review of the chemistry, pharmacology, clinical efficacy, adverse effects and costs of ropivacaine; does not mention hepatotoxicity or ALT elevations).
- Yokoyama M, Ohashi I, Nakatsuka H, Mizobuchi S, Toda Y, Matsumi M, Morita K, Hirakawa M. Drug-induced liver disease during continuous epidural block with bupivacaine. Anesthesiology 2001; 95 (1): 259-61. [PubMed: 11465567](Three women and one man, ages 62-84 years, developed fever, rash and liver test abnormalities 10-18 days after starting bupivacaine as a continuous epidural infusion [peak bilirubin 1.0-9.6 mg/dL; ALT 99-976 U/L; Alk P 233-926 U/L, eosinophilia 9-12%], resolving within one month of stopping in all patients, and not recurring in 3 patients later given lidocaine).
- Schurr MJ, Gordon DB, Pellino TA, Scanlon TA. Continuous local anesthetic infusion for pain management after outpatient inguinal herniorrhaphy. Surgery 2004; 136: 761-9. [PubMed: 15467660](Among 72 patients undergoing outpatient inguinal herniorrhaphy who received bupivacaine or placebo infusions, pain was less with the bupivacaine and side effects were minimal; no mention of ALT elevations or jaundice).
- LeBlanc KA, Bellanger D, Rhynes VK, Hausmann M. Evaluation of continuous infusion of 0.5% bupivacaine by elastomeric pump for postoperative pain management after open inguinal hernia repair. J Am Coll Surg 2005; 200: 198-202. [PubMed: 15664094](Among 44 patients undergoing hernia repair who received bupivacaine or placebo infusions, there was less pain and opiate use with therapy compared to placebo and no reports of complications).
- Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res 2012; 5: 257-64. [PMC free article: PMC3442744] [PubMed: 23049275](A liposomal preparation of bupivacaine [depo-foam] allows for a slow and prolonged release of the anesthetic from the local site of infusion and is effective in controlling postsurgical pain, but its superiority to regular bupivacaine and its safety in the general practice situation remain unclear; no mention of hepatotoxicity).
- Bupivacaine liposomal injection (Exparel) for post surgical pain. Med Lett Drugs Ther 2012; 54 (1387): 26-7. [PubMed: 22469650](Concise review of the pharmacology, clinical efficacy, safety and costs of bupivacaine liposomal injections shortly after its approval for use in postsurgical pain in the US; no mention of hepatotoxicity).
- Dickerson DM, Apfelbaum JL. Local anesthetic systemic toxicity. Aesthet Surg J 2014; 34: 1111-9. [PubMed: 25028740](Review of systemic toxicities of locally administered anesthetics which are marked early by dizziness, drowsiness, confusion and tinnitus, and which can be followed by central nervous system and cardiac abnormalities including seizures, coma, agitation, bradycardia, tachycardia, hypotension, EKG abnormalities and ventricular arrhythmias which can be severe and even fatal; no mention of hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to local anesthetics).
- Chintamaneni P, Stevenson HL, Malik SM. Bupivacaine drug-induced liver injury: a case series and brief review of the literature. J Clin Anesth 2016; 32: 137-41. [PubMed: 27290962](Three men, ages 51-66 years, developed fever, pruritus and jaundice 14-21 days after inguinal hernia repair under general anesthesia and with intra- and post-operative infusions of 98-171 mg of bupivacaine over 1-2 days [peak bilirubin 5.5-11.1 mg/dL, ALT 237-491 U/L, Alk P 365-585 U/L, eosinophils 7-9%], resolving within 1 month of onset; 2 had received single doses of cefazolin and 1 ciprofloxacin: Case 1).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Comparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitro.[Spine J. 2014]Comparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitro.Cai XY, Xiong LM, Yang SH, Shao ZW, Xie M, Gao F, Ding F. Spine J. 2014 Mar 1; 14(3):483-90. Epub 2013 Aug 23.
- Cytotoxicity of amide-linked local anesthetics on melanoma cells via inhibition of Ras and RhoA signaling independent of sodium channel blockade.[BMC Anesthesiol. 2020]Cytotoxicity of amide-linked local anesthetics on melanoma cells via inhibition of Ras and RhoA signaling independent of sodium channel blockade.Zheng Q, Peng X, Zhang Y. BMC Anesthesiol. 2020 Feb 21; 20(1):43. Epub 2020 Feb 21.
- Amide-type local anesthetics may suppress tumor cell proliferation and sensitize Human Hepatocellular Carcinoma Cells to Cisplatin via upregulation of RASSF1A expression and demethylation.[J Cancer. 2020]Amide-type local anesthetics may suppress tumor cell proliferation and sensitize Human Hepatocellular Carcinoma Cells to Cisplatin via upregulation of RASSF1A expression and demethylation.Chen D, Yan Y, Xie J, Pan J, Chen Y, Li Q, Yuan Y, Zeng W, Xing W. J Cancer. 2020; 11(24):7312-7319. Epub 2020 Oct 23.
- Review Comparison of local anesthetics for digital nerve blocks: a systematic review.[J Hand Surg Am. 2014]Review Comparison of local anesthetics for digital nerve blocks: a systematic review.Vinycomb TI, Sahhar LJ. J Hand Surg Am. 2014 Apr; 39(4):744-751.e5. Epub 2014 Mar 5.
- Review Ropivacaine. A review of its pharmacology and therapeutic use in regional anaesthesia.[Drugs. 1996]Review Ropivacaine. A review of its pharmacology and therapeutic use in regional anaesthesia.Markham A, Faulds D. Drugs. 1996 Sep; 52(3):429-49.
- Amide Local Anesthetics - LiverToxAmide Local Anesthetics - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...