NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Amantadine is a primary amine that has both antiviral and dopaminergic activity and is used in the therapy of influenza A and management of Parkinson disease. Amantadine has not been associated with clinically apparent liver injury.
Background
Amantadine (a man' ta deen) is a cyclic primary amine that has antiviral and anti-Parkinsonian activities. The antiviral activity of amantadine is attributed to inhibition of virion uncoating and release of viral RNA in the initial stages of viral replication. Amantadine is active only against influenza A virus and has no activity against influenza B or other upper respiratory viruses. In addition, resistance to amantadine (with cross resistance to rimantadine) can develop rapidly and is now common. The anti-Parkinsonian activity of amantadine appears to be due to its effects on release of dopamine in the substantia nigra. Amantadine is indicated for therapy of influenza A and in management of Parkinson disease and extrapyramidal reactions. Amantadine was approved for use in the United States in 1968 and is currently used both for influenza and Parkinson disease. Amantadine is available as capsules or tablets of 100 mg and as oral syrup generically and under the brand name of Symmetrel. The recommended oral dose for treatment of influenza in adults is 200 mg daily until symptoms have resolved; amantadine can also be administered as prophylaxis as soon as possible after close personal exposure. The dose in Parkinson disease is 100 to 400 mg daily in divided doses. Side effects include anxiety, dizziness, ataxia, confusion, fatigue, headache, insomnia, dry mouth and gastrointestinal upset. Rare potentially severe adverse events include convulsions, depression, suicidal ideation and behavior, acute psychosis, heart failure and hypersensitivity reactions.
Hepatotoxicity
Despite widespread use, there is little evidence that amantadine when given orally causes liver injury, either in the form of serum enzyme elevations or clinically apparent liver disease.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Amantadine has minimal hepatic metabolism and is excreted largely unchanged in the urine, factors which perhaps explain the absence of significant hepatotoxicity.
Drug Classes: Antiviral Agents; Antiparkinson Agents
Other Drugs in the Class for Influenza: Baloxavir, Oseltamivir, Peramivir, Rimantadine, Zanamivir
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Amantadine – Generic, Symmetrel®
DRUG CLASS
Antiviral Agents; Antiparkinson Agents
Product labeling at DailyMed, National Library of Medicine, NIH
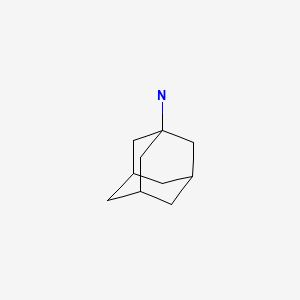
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Amantadine | 768-94-5 | C10-H17-N |
|
ANNOTATED BIBLIOGRAPHY
References updated: 22 June 2020
- Zimmerman HJ. Antiviral agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 621-3.(Expert review of antiviral agents and liver injury published in 1999; mentions that both amantadine and rimantadine have "no adverse effect on the liver").
- Núñez M. Influenza virus treatments. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 513.(Review of hepatotoxicity of antiviral agents; amantadine has been linked to rare instances of liver enzyme elevations).
- Acosta EP. Antiviral agents (nonretroviral). In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1105-18.(Textbook of pharmacology and therapeutics).
- Schnack H, Wewalka F, Guttmann G. Liver function during amantadine hydrochloride medication in compensated liver disease. Int Z Klin Pharmakol Ther Toxikol. 1969;2:185–7. [PubMed: 5800713](Placebo controlled trial of 4 weeks of amantadine in 25 patients with cirrhosis showed no difference in ALT, AST, Alk P or bilirubin levels during therapy).
- Styrt B, Freiman JP. Hepatotoxicity of antiviral agents. Gastroenterol Clin North Am. 1995;24:839–52. [PubMed: 8749901](Review of liver toxicity of antiviral agents mentions that there is little information on the potential hepatotoxicity of amantadine and the product label does not mention liver injury).
- Jefferson T, Demicheli V, Di Pietrantonj C, Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst Rev. 2006;(2):CD001169. [PMC free article: PMC7068158] [PubMed: 16625539](Systematic review of efficacy of amantadine and rimantadine in treatment and prevention of influenza A; side effects are frequent, but largely consist of dizziness, insomnia, lightheadedness and headache; no mention of liver tests abnormalities or hepatitis).
- Crosby N, Deane KH, Clarke CE. Amantadine in Parkinson's disease. Cochrane Database Syst Rev. 2003;(1):CD003468. [PMC free article: PMC8715353] [PubMed: 12535476](Systematic review of efficacy of amantadine in treatment of Parkinson disease; side effects are frequent, but largely consist of dizziness, insomnia, lightheadedness and headache; no mention of liver test abnormalities or hepatitis).
- Drugs for non-HIV viral infections. Treat Guidel Med Lett. 2007;5:59–70. [PubMed: 17565338](Review of status of non-antiretroviral antiviral agents for prevention and treatment of herpes, varicella-zoster, cytomegalovirus, influenza A and B, and hepatitis B and C; no mention of liver related side effects for amantadine).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008; 8 were attributed to antiviral agents but none to amantadine or other antiinfluenza medications).
- Antiviral drugs. Treat Guidel Med Lett. 2013;11(127):19–30. [PubMed: 23459414](Review of status of non-antiretroviral antiviral agents; because of the high rate of resistance of influenza A virus isolates to amantadine and rimantadine, they are not currently recommended either for prevention or treatment of influenza; no discussion of side effects).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no case was attributed to amantadine or any other drug for either Parkinson disease or influenza).
- Antiviral drugs for treatment and prophylaxis of seasonal influenza. Med Lett Drugs Ther. 2019;61(1563):1–4. [PubMed: 30681660](Concise review of the drug therapy of influenza mentions that there are no data suggesting the superiority of one neuraminidase inhibitor over another, they are all approved for treatment of uncomplicated influenza, should be started as soon as possible, and have been shown to shorten the duration of symptoms by one day in adults; mentions that rimantadine and amantadine are no longer recommended for treatment of influenza because of high rates of resistance in circulating strains of influenza A and they are ineffective against influenza B).
- Antiviral drugs for influenza. Med Lett Drugs Ther. 2020;62(1589):1–4. [PubMed: 31999661](Concise review of the drug therapy of influenza mentions that there is no evidence that one neuraminidase inhibitor or baloxavir is more effective than any other in treating uncomplicated influenza infections, but that oseltamivir is preferred for treatment of pregnant women, hospitalized patients and those with severe, complicated or progressive illness; mentions that rimantadine and amantadine are no longer recommended for treatment of influenza because of high rates of resistance in circulating strains of influenza A and they are ineffective against influenza B).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- An M2-V27A channel blocker demonstrates potent in vitro and in vivo antiviral activities against amantadine-sensitive and -resistant influenza A viruses.[Antiviral Res. 2017]An M2-V27A channel blocker demonstrates potent in vitro and in vivo antiviral activities against amantadine-sensitive and -resistant influenza A viruses.Hu Y, Musharrafieh R, Ma C, Zhang J, Smee DF, DeGrado WF, Wang J. Antiviral Res. 2017 Apr; 140:45-54. Epub 2017 Jan 10.
- Antiviral agents derived from novel 1-adamantyl singlet nitrenes.[Antivir Chem Chemother. 2012]Antiviral agents derived from novel 1-adamantyl singlet nitrenes.Kesel AJ, Weiss HC, Schönleber A, Day CW, Barnard DL, Detorio MA, Schinazi RF. Antivir Chem Chemother. 2012 Dec 7; 23(3):113-28. Epub 2012 Dec 7.
- Antiviral drugs in influenza: an adjunct to vaccination in some situations.[Prescrire Int. 2006]Antiviral drugs in influenza: an adjunct to vaccination in some situations.. Prescrire Int. 2006 Feb; 15(81):21-30.
- Review Emergence of amantadine-resistant influenza A viruses: epidemiological study.[J Infect Chemother. 2003]Review Emergence of amantadine-resistant influenza A viruses: epidemiological study.Suzuki H, Saito R, Masuda H, Oshitani H, Sato M, Sato I. J Infect Chemother. 2003 Sep; 9(3):195-200.
- Review Amantadine: the journey from fighting flu to treating Parkinson disease.[Neurology. 2012]Review Amantadine: the journey from fighting flu to treating Parkinson disease.Hubsher G, Haider M, Okun MS. Neurology. 2012 Apr 3; 78(14):1096-9.
- Amantadine - LiverToxAmantadine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...