NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Altretamine is an orally administered alkylating agent, formerly known as hexamethylmelamine, which is currently used as a secondary therapy for advanced ovarian carcinoma. Altretamine therapy has been associated with low rates of serum enzyme elevations during therapy and with rare instances of acute, clinically apparent injury.
Background
Altretamine (al tret' a meen) is a synthetic, orally available alkylating agent belonging to the methylmelamine class of these agents. The alkylating agents act by causing modification and cross linking of DNA, thus inhibiting DNA, RNA and protein synthesis and causing cell death in rapidly dividing cells. Altretamine was approved for use in the United States in 1990. Current indications are advanced ovarian carcinoma in patients who have failed primary therapy with platinum-containing or other alkylating agents. Altretamine is available in capsules of 50 mg under the brand name Hexalen. The recommended dose varies by indication and body surface area and it is typically given orally for 14 to 21 days in 28 day cycles. Altretamine shares common side effects with other alkylating agents such as nausea, vomiting, diarrhea, alopecia, bone marrow suppression, peripheral neuropathy and rash.
Hepatotoxicity
Altretamine therapy is associated with a low rate of serum enzyme elevations, but these are generally mild and self limited, not requiring dose adjustment. Rare instances of clinically apparent acute liver injury attributed to altretamine have been reported, but the clinical features have not been characterized. Altretamine has not been linked specifically to sinusoidal obstruction syndrome, but it is rarely used in high doses in neoplastic disease or in conditioning regimens for bone marrow transplantation, situations in which alkylating agents are commonly associated with this complication.
Likelihood score: E* (unlikely but suspected rare cause of liver injury).
Mechanism of Injury
The cause of the idiosyncratic liver injury associated with altretamine use is probably related to a hypersensitivity reaction to a hepatic metabolite of the drug, which is extensively metabolized by the liver.
Outcome and Management
Liver injury is very rare after altretamine therapy. The severity of injury in reported cases has generally been mild-to-moderate and self limited in course. There have been no instances of acute liver failure, chronic hepatitis or vanishing bile duct syndrome definitely linked to chlorambucil therapy. In situations of acute liver injury after altretamine use, rechallenge should be avoided.
Drug Class: Antineoplastic Agents, Alkylating Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Altretamine – Hexalen®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Altretamine | 645-05-6 | C9-H18-N6 |
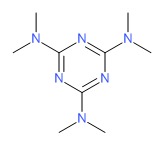 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 March 2016
- Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; triethylenemelamine but not altretamine is discussed).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; altretamine is not discussed).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1315-404.(Textbook of pharmacology and therapeutics).
- Ihde DC, Dutcher JS, Young RC, Cordes RS, Barlock AL, Hubbard SM, Jones RB, et al. Phase I trial of pentamethylmelamine: a clinical and pharmacologic study. Cancer Treat Rep 1981; 65: 755-62. [PubMed: 6791819](Phase 1 study of a derivative of altretamine in 34 patients with advanced solid tumors given in 1-24 intravenous infusions; 9 patients had elevations in ALT or AST, but no jaundice and most abnormalities occurred in patients with liver metastases).
- Rollins BJ. Hepatic veno-occlusive disease. Am J Med 1986; 8: 297-306. [PubMed: 3526887](Review of the diagnosis, clinical course, histology and pathogenesis of veno-occlusive disease [sinusoidal obstruction syndrome: SOS]).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation 1987; 4: 778-83. [PubMed: 3321587](Among 235 patients undergoing bone marrow transplantation between 1982 and 1985, sinusoidal obstruction syndrome [SOS] developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Manetta A, MacNeill C, Lyter JA, Scheffler B, Podczaski ES, Larson JE, Schein P. Hexamethylmelamine as a single second-line agent in ovarian cancer. Gynecol Oncol 1990; 36: 93-6. [PubMed: 2104819](Among 52 patients with advanced ovarian cancer given altretamine in cycles of 14 days, side effects were mainly nausea, vomiting and sensory neuropathy; Alk P elevations occurred in 7 patients, but no details given).
- Ames MM. Hexamethylmelamine: pharmacology and mechanism of action. Cancer Treat Rep 1991; 18: 3-14. [PubMed: 1904306](Pharmacology of altretamine demonstrates variable absorption orally, dose related toxicity is nausea and vomiting; activity requires metabolic activation in the liver after which it binds to macromolecules).
- Lee CR, Faulds D. Altretamine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cancer chemotherapy. Drugs 1995; 49: 932-53. [PubMed: 7641606](Review of pharmacology, clinical efficacy and safety of altretamine; no mention of hepatotoxicity).
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005-20. [PubMed: 7756636](Review of sinusoidal obstruction syndrome after hematopoietic cell transplantation; usually presents with painful hepatomegaly, weight gain [fluid and ascites] and jaundice within 3 weeks of myeloablation with occlusion of central veins and sinusoids and extensive zone 3 [centrolobular] injury).
- Malik IA. Altretamine is an effective palliative therapy of patients with recurrent epithelial ovarian cancer. Jpn J Clin Oncol 2001; 31: 69-73. [PubMed: 11302345](Among 17 women with recurrent ovarian cancer treated with altretamine for an average of 6 months, toxicity was mainly nausea, vomiting and asthenia; 5 patients developed "Abdominal LFT", but no patient was withdrawn because of toxicity and no details were provided on ALT elevations or hepatotoxicity).
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Altretamine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cancer chemotherapy.[Drugs. 1995]Review Altretamine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cancer chemotherapy.Lee CR, Faulds D. Drugs. 1995 Jun; 49(6):932-53.
- Altretamine (hexamethylmelamine) in platinum-resistant and platinum-refractory ovarian cancer: a Gynecologic Oncology Group phase II trial.[Gynecol Oncol. 1998]Altretamine (hexamethylmelamine) in platinum-resistant and platinum-refractory ovarian cancer: a Gynecologic Oncology Group phase II trial.Markman M, Blessing JA, Moore D, Ball H, Lentz SS. Gynecol Oncol. 1998 Jun; 69(3):226-9.
- [Combination chemotherapy with hexamethylamine (Hexalen, Altretamine, Hexastat) and sarcolysine in advanced ovarian carcinoma].[Vopr Onkol. 2000][Combination chemotherapy with hexamethylamine (Hexalen, Altretamine, Hexastat) and sarcolysine in advanced ovarian carcinoma].Gershanovich ML, Livshits ME, Antipenkova VI. Vopr Onkol. 2000; 46(5):604-7.
- Hexamethylmelamine in alkylating agent-resistant ovarian carcinoma.[Cancer. 1978]Hexamethylmelamine in alkylating agent-resistant ovarian carcinoma.Johnson BL, Fisher RI, Bender RA, DeVita VT Jr, Chabner BA, Young RC. Cancer. 1978 Nov; 42(5):2157-61.
- Review Hexamethylmelamine: activity in lymphoma and other tumors.[Cancer Treat Rev. 1991]Review Hexamethylmelamine: activity in lymphoma and other tumors.Macdonald JS. Cancer Treat Rev. 1991 Mar; 18 Suppl A:99-102.
- Altretamine - LiverToxAltretamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...