NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Aliskiren is a unique antihypertensive agent that acts by direct inhibition of renin and has only recently been introduced into clinical practice. Aliskiren has been linked to rare instances of serum aminotransferase elevations during therapy and rare cases of idiosyncratic, clinically apparent liver injury.
Background
Aliskiren (al' is key' ren) is a direct inhibitor of renin and acts to inhibit its ability to convert angiotensinogen to angiotensin 1, an early step in the renin-angiotensin system pathway. By inhibiting renin and the subsequent production of angiotensin II and aldosterone release, aliskiren results in a decrease in peripheral vasoconstriction and increase in sodium excretion. Aliskiren is effective in lowering blood pressure and can be used alone or in combination with other antihypertensive medications, including other inhibitors of the renin-angiotensin system such as the angiotensin converting enzyme (ACE) inhibitors and the angiotensin receptor blockers (ARBs). In 2007, aliskiren became the first in the class of direct renin inhibitors to be approved for use in the United States. Aliskiren is available in under the brand name of Tekturna in tablets of 150 and 300 mg. Fixed combinations of aliskiren with hydrochlorthiazide (Tekturna HCT), valasartan (Valturna) and amlodipine (Amturnide) are also available. The typical initial dose of aliskiren in adults is 150 daily, which can be increased to 300 mg daily based upon effects and tolerance. Side effects are uncommon and usually mild; they include diarrhea, headaches, fatigue, dizziness and nasopharyngitis.
Hepatotoxicity
Serum aminotransferase elevations during aliskiren therapy are uncommon and rates of such elevations have not been reported in the large clinical trials that demonstrated its efficacy in hypertension. An instance of serum aminotransferase elevation with jaundice was reported in the registration trials of aliskiren and several instances of marked serum enzyme elevations during therapy with few symptoms and without jaundice have been reported. In most instances, the pattern of enzyme elevation was distinctly hepatocellular and recovery was prompt once aliskiren was stopped. There have also been reports to the sponsor of severe hepatic reactions including hepatic failure and more recently a published case report of acute liver injury attributed to this agent. The latency to onset has ranged from 1 to 6 months and the pattern of injuryis typically cholestatic or mixed. Most cases have been mild to moderate in severity and marked by rapid recovery once aliskiren is stopped.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of liver injury due to aliskiren is not known. Aliskiren is metabolized in the liver by the cytochrome P450 (CYP 3A4) system, but has little effect on drug metabolizing activity. Concurrent use of CYP 3A4 inhibitors such as cyclosporin or itraconazole can cause an increase in aliskiren levels.
Outcome and Management
Severity ranges from asymptomatic elevations in serum aminotransferase levels to mildly symptomatic cholestatic hepatitis with or without jaundice. There are no convincing cases of fulminant hepatic failure, chronic hepatitis or vanishing bile duct syndrome attributable to aliskerin use in the published literature. Patients with aliskiren induced liver injury need not avoid other antiihypertensive agents.
Drug Class: Antihypertensive Agents
CASE REPORTS SUBMITTED TO LIVERTOX
Clinical cases of drug-induced liver injury that have been submitted to LiverTox ("Submit a Case Report") are available for review. Most of these reference cases are from the Drug-Induced Liver Injury Network, but others are from users of LiverTox who have submitted data from an actual clinical case. All cases have been reviewed and cleared of personal identifiers and a brief comment added by the LiverTox editors. Click on the following link to view the submitted case reports that have been made publically available.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Aliskiren – Tekturna®
DRUG CLASS
Antihypertensive Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Aliskiren | 173334-57-1 | C30-H53-N3-O6 |
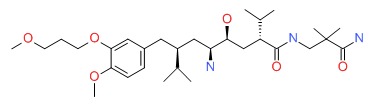 |
ANNOTATED BIBLIOGRAPHY
References updated: 23 February 2016
- Zimmerman HJ. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 655-6.(Expert review of hepatotoxicity published in 1999, before the availability of aliskiren).
- De Marzio DH, Navarro VJ. Antihypertensives. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 522-6.(Review of liver injury due to antihypertensive agents; aliskiren is not discussed).
- Hilal-Dandan R. Renin and Angiotensin. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 721-44.(Textbook of pharmacology and therapeutics).
- Michel T, Hoffman BB. Therapy of myocardial ischemia and hypertension. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 745-88.(Textbook of pharmacology and therapeutics; aliskiren has a place as an add-on rather than a first line drug in treating hypertension).
- Straessen JA, Li Y, Richart T. Oral renin inhibitors. Lancet 2006; 368: 1449-56. [PubMed: 17055947](Review of history of attempts to develop direct renin inhibitors and the pharmacology and mechanism of action of aliskiren; no discussion of side effects or hepatotoxicity).
- Daugherty KK. Aliskiren. Am J Health Syst Pharm 2008; 65: 1323-32. [PubMed: 18593678](Review of pharmacology, pharmacokinetics, clinical efficacy and safety of aliskiren; most common side effects were headache, fatigue, dizziness and diarrhea [all <10%, and similar to rates with placebo]; rare side effects [<1%] were rash, gout, and renal stones, no mention of ALT elevations or hepatotoxicity).
- Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005; 111: 1012-8. [PubMed: 15723979](Controlled trial of 8 weeks of aliskiren vs irbesartan vs placebo in 652 patients with hypertension; side effects were similar in the 3 groups and laboratory values remained normal “in the majority of patients”, but one patient on aliskiren stopped treatment because of elevations in AST and bilirubin).
- Cheng JW. Aliskiren: renin inhibitor for hypertension management. Clin Ther 2008; 30: 31-47. [PubMed: 18343241](Systematic review of structure, pharmacology, mechanism of action, clinical efficacy and side effects of aliskiren; side effects evaluated in more than 6000 patients; most common included headache, dizziness, fatigue and gastrointestinal; no mention of ALT elevations or hepatotoxicity).
- Luft FC, Weinberger MH. Antihypertensive therapy with aliskiren. Kidney Int 2008; 73: 679-83. [PubMed: 18160962](Review of pharmacology and mechanism of action of aliskiren).
- Drugs for hypertension. Treat Guidel Med Lett 2009; 7: 1-10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use with information on prices and toxicities; aliskiren is said to have similar side effects to the angiotensin receptor blockers, but also can cause gastrointestinal effects such as diarrhea).
- Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, Maboudian M, et al. Long-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation 2009; 119: 417-25. [PubMed: 19139391](Controlled trial of aliskiren vs hydrochlorothiazide [HCTZ] vs placebo in 1124 patients with hypertension; adverse event rates were similar in the aliskiren and HCTZ groups and were generally mild and transient; no mention of ALT elevations or hepatotoxicity and no deaths attributed to drug therapy).
- Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL. Long-term safety, tolerability, and antihypertensive efficacy of aliskiren, an oral direct rennin inhibitor, in Japanese patients with hypertension. Hypertens Res 2009; 32: 169-75. [PubMed: 19262478](Open label study of 52 weeks of aliskiren in 345 patients with hypertension; side effects were mild and rarely required dose modification; no mention of ALT elevations or hepatotoxicity).
- Angeli F, Reboldi G, Mazzotta G, Poltronieri C, Verdecchia P. Safety and efficacy of aliskiren in the treatment of hypertension: a systematic overview. Expert Opin Drug Saf 2012; 11: 659-70. [PubMed: 22724663](Aliskiren is well tolerated and side effects are "placebo-like", the only symptom more frequent with aliskiren being diarrhea [2.3%]; no discussion of ALT elevations or hepatotoxicity).
- Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, et al.; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204-13. [PubMed: 23121378](Among 8561 patients with diabetes and either renal or cardiovascular disease, the addition of aliskiren to chronic therapy an ACE inhibitor or angiotensin receptor blocker was associated with higher rates of side effects and no decrease in cardiovascular complications; no mention of ALT elevations or hepatotoxicity).
- Crepin S, Godet B, Carrier P, Villeneuve C, Merle L, Laroche ML. Probable drug-induced liver injury associated with aliskiren: case report and review of adverse event reports from pharmacovigilance databases. Am J Health Syst Pharm 2014; 71: 643-7. [PubMed: 24688038](61 year old woman was found to have elevations in serum enzymes one month after starting aliskiren [bilirubin 0.5 mg/dL, ALT 987 U/L, Alk P 466 U/L, GGT 820 U/L], with rapid improvement on stopping; review of various adverse event registries identified 272 reports of hepatic events, mostly serum enzyme elevations only, but at least 6 of "hepatic failure").
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to aliskiren; a 65 year old woman developed liver enzyme elevations with minimal symptoms 7 months after starting aliskiren [bilirubin 0.6 mg/dL, ALT 545 U/L, Alk P 239 U/L], resolving within 6 months of stopping).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Long-term safety, tolerability and efficacy of aliskiren in combination with valsartan in patients with hypertension: a 6-month interim analysis.[Curr Med Res Opin. 2008]Long-term safety, tolerability and efficacy of aliskiren in combination with valsartan in patients with hypertension: a 6-month interim analysis.Chrysant SG, Murray AV, Hoppe UC, Dattani D, Patel S, Hsu H, Zhang J. Curr Med Res Opin. 2008 Apr; 24(4):1039-47. Epub 2008 Feb 27.
- Review Aliskiren: an oral direct renin inhibitor for the treatment of hypertension.[Pharmacotherapy. 2009]Review Aliskiren: an oral direct renin inhibitor for the treatment of hypertension.Sanoski CA. Pharmacotherapy. 2009 Feb; 29(2):193-212.
- Review Aliskiren, a direct renin inhibitor, in clinical practice: a new approach in the treatment of hypertension.[Curr Vasc Pharmacol. 2010]Review Aliskiren, a direct renin inhibitor, in clinical practice: a new approach in the treatment of hypertension.Moutzouri E, Florentin M, Elisaf MS, Mikhailidis DP, Liberopoulos EN. Curr Vasc Pharmacol. 2010 May; 8(3):344-62.
- Aliskiren attenuates chronic carbon tetrachloride-induced liver injury in mice.[Eur J Clin Invest. 2012]Aliskiren attenuates chronic carbon tetrachloride-induced liver injury in mice.Lee KC, Chan CC, Yang YY, Hsieh YC, Huang YH, Lin HC. Eur J Clin Invest. 2012 Dec; 42(12):1261-71. Epub 2012 Sep 23.
- Review Clinical pharmacokinetics and pharmacodynamics of aliskiren.[Clin Pharmacokinet. 2008]Review Clinical pharmacokinetics and pharmacodynamics of aliskiren.Vaidyanathan S, Jarugula V, Dieterich HA, Howard D, Dole WP. Clin Pharmacokinet. 2008; 47(8):515-31.
- Aliskiren - LiverToxAliskiren - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...