NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Afatinib is a tyrosine kinase receptor inhibitor that is used in the therapy of selected forms of metastatic non-small cell lung cancer. Afatinib is associated with transient elevations in serum aminotransferase levels during therapy and has been reported to cause clinically apparent acute liver injury and rare instances of death.
Background
Afatinib (a fa' ti nib) is a small molecule tyrosine kinase receptor inhibitor with potent activity against epidermal growth factor receptor 2 (EGFR2 or ErbB1) and human epidermal growth factor receptor 2 (HER2 or ErbB2). These tyrosine kinase receptors are often mutated and over expressed in tumor tissue and cause unregulated cell growth and proliferation. Inhibition of the unregulated receptor can lead to reversal of progression of the cancer, although clinical responses are sometimes limited by the development of tumor resistance caused by further mutations in the receptor gene. In several large controlled trials, afatinib was shown to improve progression-free survival in patients with non-small cell lung cancer (NSCLC) with mutated EGFR2. Afatinib has also shown promise in therapy of some cases of advanced breast cancer. Afatinib received approval for use in the United States in 2013. Current indications are as primary therapy for metastatic NSCLC with EGFR2 mutations and as second line therapy in patients with previously treated and refractory squamous cell lung cancer. Afatinib is available in tablets of 20, 30 and 40 mg under the brand name Gilotrif. The recommended initial dose is 40 mg once daily, continued until disease progresses or intolerable toxicity occurs. Side effects are common and include diarrhea, rash, stomatitis, anorexia, nausea, dry skin, paronychia and pruritus. Uncommon, but potentially severe side effects include severe diarrhea leading to dehydration and renal failure, bullous and exfoliative skin rashes, Stevens Johnson syndrome, interstitial lung disease and embryo-fetal toxicity.
Hepatotoxicity
Elevations in serum aminotransferase levels are common during afatinib therapy occurring in 20% to 50% of patients, but rising above 5 times the upper limit of the normal range in only 1% to 2%. Hepatic failure is said to have occurred in 0.2% of patients and to have resulted in several fatalities. Hepatotoxicity appears to be a class effect among protein kinase inhibitors of EGFR2, although liver injury appears to be more frequent and more severe with gefitinib than with afatinib and erlotinib. Specific details of the liver injury associated with afatinib such as latency, serum enzyme pattern, clinical features and course, have not been published. Other EGFR inhibitors, such as erlotinib and gefitinib typically cause liver injury arising within days or weeks of starting therapy and presenting abruptly with hepatocellular enzyme elevations and a moderate-to-severe course. Immunoallergic and autoimmune features are not common. The rate of clinically significant liver injury and hepatic failure is increased in patients with preexisting cirrhosis or hepatic impairment due to liver tumor burden.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The abrupt and severe nature of the clinically apparent liver injury attributed to EGF receptor inhibitors suggests that it is immunologically mediated. However, the transient serum enzyme elevations that are not uncommon during therapy suggest a direct, intrinsic hepatotoxicity, perhaps caused by inhibition of critical tyrosine kinase receptors in hepatocytes. Afatinib is metabolized in the liver largely and is susceptible to drug-drug interactions with inhibitors or inducers of p-glycoprotein.
Outcome and Management
Liver injury due to afatinib varies in severity from minor, transient serum enzyme elevations to acute symptomatic hepatitis and acute liver failure. Periodic monitoring of liver tests during afatinib therapy is recommended. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to temporary discontinuation, which should be permanent if laboratory values do not improve significantly or resolve within a few weeks, or if symptoms or jaundice arises. Corticosteroids have been used in some instances of acute liver injury due to EGFR inhibitors. Restarting therapy is usually, but not always followed by recurrence of the serum enzyme elevations. There does not appear to be cross reactivity with other tyrosine kinase receptor inhibitors and, in some situations, switching to another protein kinase inhibitor may be appropriate.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Afatinib – Gilotrif®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
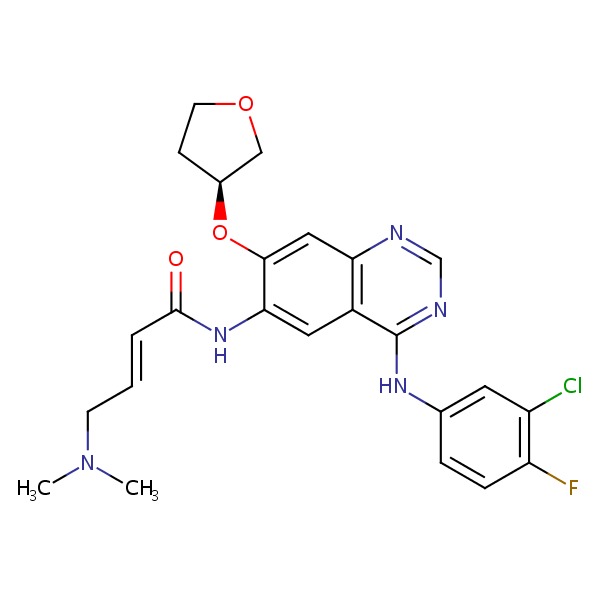
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Afatinib | 850140-72-6 | C24-H25-Cl-F-N5-O3 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 June 2017
Abbreviations used: NSCLC, non-small cell lung cancer; HER-2, human epidermal growth factor receptor-2; EGFR, epidermal growth factor receptor.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Kinase inhibitorsb. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556-7.(Review of hepatotoxicity of cancer chemotherapeutic agents; discusses liver injury attributed to tyrosine kinase inhibitors gefitinib, erlotinib and crizotinib but not afatinib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- Schacher-Kaufmann S, Pless M. Acute fatal liver toxicity under erlotinib. Case Rep Oncol 2010; 3: 182-8. [PMC free article: PMC2919997] [PubMed: 20740194](53 year old with non-small cell lung cancer developed anorexia 9 days and abdominal pain and fever 17 days after starting erlotinib [bilirubin 4.7 mg/dL, ALT 884 U/L, Alk P 814 U/L], with progressive liver failure and thrombotic microangiopathy, dying 2 weeks after stopping erlotinib).
- Gunturu KS, Abu-Khalaf M, Saif MW. Hepatic failure and hepatorenal syndrome secondary to erlotinib: a possible etiology of complications in a patient with pancreatic cancer. JOP 2010; 11: 484-5. [PubMed: 20818124](A 39 year old man with advanced pancreatic cancer developed weakness and rash after 3 days of erlotinib therapy [bilirubin 4.0 rising to 17.2 mg/dL, ALT 489 U/L], rapidly progressing to liver failure associated with diffuse liver metastases and malignant ascites).
- Lai YC, Lin PC, Lai JI, Hsu SY, Kuo LC, Chang SC, Wang WS. Successful treatment of erlotinib-induced acute hepatitis and acute interstitial pneumonitis with high-dose corticosteroid: a case report and literature review. Int J Clin Pharmacol Ther 2011; 49: 461-6. [PubMed: 21726497](A 74 year old man developed interstitial pneumonitis and mild serum enzyme elevations one month after starting erlotinib [ALT 116 U/L], both of which improved within days on high dose corticosteroids).
- Schuler M, Awada A, Harter P, Canon JL, Possinger K, Schmidt M, De Grève J, et al. A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat 2012; 134: 1149-59. [PMC free article: PMC3409367] [PubMed: 22763464](Among 50 women with HER2-negative metastatic breast cancer treated with afatinib, there were no objective responses and 96% of patients had adverse events which were serious in 40%; no mention of liver related events).
- Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy(LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012; 13: 528-38. [PubMed: 22452896](Among 585 patients with previously treated metastatic NSCLC treated with afatinib or placebo, progression-free survival was 3.3 months with afatinib and 1.1 with placebo, while adverse events included diarrhea in 87% and acneiform rash in 78%; no mention of liver related adverse events).
- Yoshida T, Yamada K, Azuma K, Kawahara A, Abe H, Hattori S, Yamashita F, et al. Comparison of adverse events and efficacy between gefitinib and erlotinib in patients with non-small-cell lung cancer: a retrospective analysis. Med Oncol 2013; 30: 349. [PubMed: 23263831](Comparison of side effects in 107 patients with NSCLC treated with gefitinib and 35 with erlotinib found "liver dysfunction" more frequent with gefitinib [13% vs 6%], whereas overall adverse events leading to drug discontinuation were less common [13% vs 26%]).
- Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327-34. [PubMed: 23816960](Among 345 patients with metastatic NSCLC with EGFR mutations treated with afatinib or cyclic chemotherapy with cisplatin and pemetrexed, progression-free survival was longer with afatinib [11.1 vs 6.9 months]; side effects were common, but no mention of ALT elevations or hepatotoxicity).
- Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213-22. [PubMed: 24439929](Among 364 patients with EGFR-mutant NSCLC treated with afatinib [40 mg daily] or gemcitabine and cisplatin [6 3-week cycles], median progression free survival was longer with afatinib [11 vs 5.6 months], while overall and serious adverse event rates were similar; ALT elevations arising in 20% vs 16% and levels above 5 times ULN in 1.7% vs 2.7%, but no mention of clinically apparent liver injury).
- Cortés J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, Le Rhun E, et al. Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol 2015; 16: 1700-10. [PubMed: 26596672](Among 121 women with advanced HER-2 positive breast cancer treated with afatinib alone, afatinib and vinorelbine, or standard of care chemotherapy, overall and progression-free survival were similar in the three groups, but afatinib was associated with more frequent adverse events and discontinuations [43% and 43% vs 7%]; no mention of ALT elevations or clinically apparent liver injury).
- Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, Göker E, et al.; LUX-Lung 8 Investigators. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015; 16: 897-907. [PubMed: 26156651](Among 795 patients with advanced, squamous cell cancer of the lung treated with afatinib or erlotinib, progression-free survival was longer with afatinib [2.4 vs 1.9 months], while overall [99% vs 97%] and serious [44% vs 44%] adverse event rates were similar; ALT elevations above 5 times ULN occurred in 1.1% vs 0.3%, but no mention of clinically apparent liver injury).
- Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015; 88: 74-9. [PubMed: 25704957](A pooled analysis of 21 trials of tyrosine kinase inhibitors of EGFR found higher reported rates of "grade 3" hepatotoxicity with gefitinib [18%] than with erlotinib [5.4%] and afatinib [1.7%], and hepatotoxicity was listed as the cause of drug discontinuation in 25% of cases).
- Afatinib (Gilotrif) for advanced non-small cell lung cancer. Med Lett Drugs Ther 2015; 57 (1469): e82-3. [PubMed: 26039556](Concise review of the mechanism of action, clinical efficacy, safety and costs of afatinib shortly after its approval in the US as therapy of metastatic NSCLC; mentions that there have been fatalities from hepatic toxicity).
- Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141-51. [PubMed: 25589191](Pooled analysis of two trials of afatinib vs standard chemotherapies for NSCLC found similar overall survival rates; no mention of ALT elevations or liver toxicity from afatinib).
- Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016; 17: 577-89. [PubMed: 27083334](Among 319 patients with EGFR mutant positive NSCLC treated with afatinib [40 mg] or gefitinib [250 mg] daily, progression- free survival was similar in both groups [11.0 vs 10.9 months] as were overall rates of adverse events, although ALT or AST elevations were less frequent with afatinib [all elevations 10% vs 24%, elevations above 5 times ULN 0% vs 1%] and one patient on gefitinib developed fatal acute liver failure).
- Toba H, Sakiyama S, Takizawa H, Tangoku A. Safe and successful treatment with afatinib in three postoperative non-small cell lung cancer patients with recurrences following gefitinib/erlotinib-induced hepatotoxicity. J Med Invest 2016; 63: 149-51. [PubMed: 27040072](Three patients with metastatic NSCLC who developed ALT elevations during gefitinib therapy [316, 414 and 687 U/L], one of whom also had ALT elevations with erlotinib [442 U/L] later tolerated afatinib treatment without recurrence of liver test abnormalities).
- Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, Yang JC, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non-small cell lung cancer. J Thorac Oncol 2017; 12 (4): 633-43. [PubMed: 28007626](In a systematic review of 16 trials of EGFR tyrosine kinase inhibitors, liver enzyme elevations were highest for gefitinib [62%] vs afatinib [20%] and erlotinib [18%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Alectinib.[LiverTox: Clinical and Researc...]Review Alectinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Erlotinib.[LiverTox: Clinical and Researc...]Review Erlotinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Brigatinib.[LiverTox: Clinical and Researc...]Review Brigatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Crizotinib.[LiverTox: Clinical and Researc...]Review Crizotinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Osimertinib.[LiverTox: Clinical and Researc...]Review Osimertinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Afatinib - LiverToxAfatinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...