NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Acebutolol is a cardioselective beta-blocker used in the treatment of hypertension, angina pectoris and cardiac arrhythmias. Acebutolol has been linked to several instances of clinically apparent drug induced liver injury.
Background
Acebutolol (a" se bue' toe lol) is considered a “selective” beta-adrenergic receptor blocker in that it has potent activity against beta-1 adrenergic receptors which are found in cardiac muscle, but has little or no activity against beta-2 adrenergic receptors found on bronchial and vascular smooth muscle. Acebutolol also has mild sympathomimetic activity (a partial adrenergic agonist, similar to pinolol). Acebutolol was approved for use in the United States in 1985 and is currently used in the therapy of hypertension alone or in combination with other agents. It is also used for cardiac arrhythmias. Acebutolol is available in capsules of 200 and 400 mg in generic forms and under the trade name Sectral. The usual initial oral dose of acebutolol in adults is 200 mg once or twice daily, with subsequent adjustment based upon clinical response and tolerance; the usual maintenance dosage being 200 to 1200 mg daily. Common side effects include bradycardia, hypotension, fatigue, dizziness, depression, insomnia, memory loss and impotence. In high doses, acebutolol is less cardioselective and can induced acute bronchospasm. As with other beta-blockers, sudden withdrawal can trigger rebound hypertension.
Hepatotoxicity
Acebutolol is associated with a low rate of mild-to-moderate elevations of serum aminotransferase levels during treatment. These enzyme elevations are usually asymptomatic and transient and resolve even with continuation of therapy. There have been few documented cases of clinically apparent, acute liver injury attributed to acebutolol. The time to onset of injury was typically between 1 and 6 weeks of starting. The pattern of liver enzyme elevations was usually hepatocellular with an acute hepatitis-like presentation, although some cases present with a mixed pattern of enzymes. Fever commonly accompanied the liver injury, but usually without rash and eosinophia. Acebutolol is known to induce autoantibodies such as antinuclear antibody in 10% to 30% of patients, some of whom develop a lupus-like syndrome with fatigue, skin rash and arthralgias. Serum enzyme elevations may accompany this syndrome, but jaundice and symptoms of liver injury are uncommon. The published cases of hepatotoxicity due to acebutolol were relatively mild, self-limited and recovery was rapid upon stopping. Rechallenge resulted in rapid recurrence of injury.
Likelihood score: C (probable cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of drug induced liver injury from beta-blockers such as acebutolol is not known. Acebutolol is extensively metabolized by the liver and excreted as inactive metabolites. The few cases of clinically apparent liver injury attributed to acebutolol were likely idiosyncratic.
Outcome and Management
The severity of liver injury due to beta-blockers ranges from mild serum aminotransferase elevations to acute hepatitis with jaundice. Acebutolol has not been implicated in cases of acute liver failure or vanishing bile duct syndrome. In large case series of drug induced liver injury and acute liver failure due to medications, acebutolol has not been listed as a potential cause. There is little information about cross reactivity among the beta-blockers to hepatic injury. Switching from a beta-blocker that has caused acute liver injury to another should be done with caution and active monitoring.
References to the safety and potential hepatotoxicity of acebutolol are provided in the overview on Beta-Adrenergic Receptor Antagonists, last updated in June 2019.
Drug Class: Beta-Adrenergic Receptor Antagonists
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Acebutolol – Generic, Sectral®
DRUG CLASS
Beta-Adrenergic Receptor Antagonists
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Acebutolol | 37517-30-9 | C18-H28-N2-O4 |
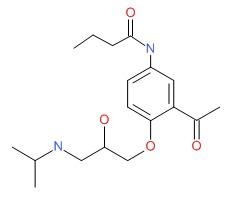 |
- PubChem SubstanceRelated PubChem Substances
- Pulmonary effects of acebutolol, a "cardioselective" beta adrenergic blocking agent.[Int J Clin Pharmacol Ther Toxi...]Pulmonary effects of acebutolol, a "cardioselective" beta adrenergic blocking agent.DiBianco R, Dickie KJ, Singh S, Katz RJ, Costello RB, Gottdiene JS, Laddu AR, Fletcher RD. Int J Clin Pharmacol Ther Toxicol. 1982 Jan; 20(1):1-7.
- Review Acebutolol. A review of its pharmacological properties and therapeutic efficacy in hypertension, angina pectoris and arrhythmia.[Drugs. 1985]Review Acebutolol. A review of its pharmacological properties and therapeutic efficacy in hypertension, angina pectoris and arrhythmia.Singh BN, Thoden WR, Ward A. Drugs. 1985 Jun; 29(6):531-69.
- Review Acebutolol: a review of its pharmacology, pharmacokinetics, clinical uses, and adverse effects.[Pharmacotherapy. 1986]Review Acebutolol: a review of its pharmacology, pharmacokinetics, clinical uses, and adverse effects.Singh BN, Thoden WR, Wahl J. Pharmacotherapy. 1986 Mar-Apr; 6(2):45-63.
- Low-dose acebutolol given once daily in the treatment of chronic angina pectoris.[J Clin Pharmacol. 1988]Low-dose acebutolol given once daily in the treatment of chronic angina pectoris.Piña IL, Smith EV, Weidler DJ. J Clin Pharmacol. 1988 May; 28(5):427-30.
- Glucose and lipid metabolism during acebutolol and propranolol therapy of angina in nondiabetic patients.[Clin Pharmacol Ther. 1983]Glucose and lipid metabolism during acebutolol and propranolol therapy of angina in nondiabetic patients.Birnbaum J, DiBianco R, Becker KL, Muesing R, Costello RB, Singh SN, Gottdiener JS, Fletcher RD. Clin Pharmacol Ther. 1983 Mar; 33(3):294-300.
- Acebutolol - LiverToxAcebutolol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...