NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Abemaciclib is a unique cyclin-dependent kinase inhibitor that is used in combination with an antiestrogen in the treatment of postmenopausal women with metastatic breast cancer. Abemaciclib is associated with a moderate rate of serum aminotransferase elevations during therapy and is suspected to be a rare cause of clinically apparent liver injury.
Background
Abemaciclib (a bem" a sye' klib) is an orally available, small molecule inhibitor of cyclin-dependent kinases (CDK) 4 and 6 that is used in combination with fulvestrant in the therapy of postmenopausal women with metastatic breast cancer that is positive for the estrogen receptor (ER) but negative for human epidermal growth factor receptor 2 (HER2). The cyclin kinases 4 and 6 regulate the cellular transition from the G1 to the S phase of the cell cycle acting through the retinoblastoma protein (Rb) pathway. Inhibition of this transition blocks the progression of the cell cycle and results in growth arrest in rapidly dividing cells. Components of this pathway are often mutated or overexpressed in cancer cells. In several clinical trials, the addition of abemaciclib to fulvestrant (a steroidal antiestrogen) therapy of metastatic breast cancer (ER+, HER2-) in postmenopausal women was associated with a prolongation of disease-free survival. As monotherapy, abemaciclib was also found effective in adult patients with refractory, metastatic HR+, HER2- breast cancer. Abemaciclib received accelerated approval for these indications in the United States in 2017, the third CDK 4/6 inhibitor approved as therapy of breast cancer, following palbociclib (Ibrance: 2015) and ribociclib (Kisqali: 2017). Abemaciclib is also under investigation as therapy of several solid tumors and lymphomas, but the initial indications were limited to metastatic ER+, HER2- breast cancer in postmenopausal women. Abemaciclib is available in tablets of 50, 100, 150 and 200 mg under the brand name Verzenio, and the initial recommended dose is 150 mg twice daily in combination with fulvestrant or 200 mg twice daily if used alone, continued indefinitely or until there is disease progression or unacceptable toxicity. Common side effects include diarrhea, neutropenia, fatigue, nausea, anorexia, thrombocytopenia, headache and back pain. Less common but potentially severe adverse reactions include venous thromboembolism, severe neutropenia, fever, neutropenic sepsis, infections and embryo-fetal toxicity.
Hepatotoxicity
In the large clinical trials, adverse events were common and led to dose reductions in up to one-half of patients and discontinuation in 9%. In preregistration clinical trials, ALT elevations occurred in 31% to 41% of abemaciclib treated subjects which were above 5 times the ULN in 3% to 5%. In one study, several recipients developed clinically apparent liver injury with jaundice and one recipient died of hepatic failure, but these outcomes were considered to be unrelated to abemaciclib therapy. Thus, there were no cases of clinically apparent liver injury that could be attributed to abemaciclib therapy during prelicensure studies. Since the approval and more widescale use of abemaciclib, there have been no published reports of its hepatotoxicity. Nevertheless, the high rate of serum enzyme elevations during therapy and the similarity of abemaciclib to ribociclib and palbociclib makes it an agent that should be suspected of causing rare instances of clinically significant liver injury.
Likelihood score: E* (unproved but suspected, rare cause of clinically apparent liver injury).
Mechanism of Injury
The causes of serum enzyme elevations and liver injury from abemaciclib therapy are not known. Abemaciclib is extensively metabolized in the liver largely through the CYP 3A4 pathway and liver injury might be caused by production of a toxic or immunogenic intermediate. On the other hand, inhibition of cyclin-dependent kinases 4 and 6 may also affect hepatocytes and have direct toxicity. Because it is a substrate for CYP 3A4, abemaciclib is susceptible to drug-drug interactions with agents that inhibit or induce this specific hepatic microsomal activity.
Outcome and Management
The product label for abemaciclib recommends prospective monitoring of liver tests during therapy, with values obtained before starting, at 2-week intervals during the first two cycles and at the beginning of the ensuing 4 cycles, and "as clinically indicated" thereafter. The product label also provides careful recommendations for dose interruption, reduction or discontinuation based upon toxicities (neutropenia, liver injury, QTc prolongation), with dose interruption until recovery for aminotransferase elevations above 3 times ULN, interruption until normal and subsequent dose reduction if above 5 times ULN, and permanent discontinuation if above 20 times ULN or in the presence of ALT elevations and jaundice. There is no information regarding cross reactivity in risk for adverse events, hypersensitivity or hepatic injury between abemaciclib and ribociclib or palbociclib or other cyclin-dependent kinase inhibitors.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Other Cyclin-Dependent Kinase Inhibitor Drugs: Palbociclib, Ribociclib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Abemaciclib – Verzenio®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
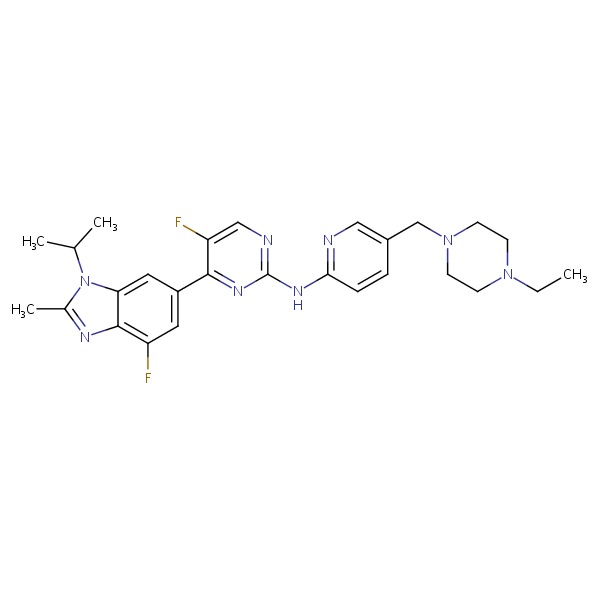
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Abemaciclib | 1231929-97-7 | C27-H32-F2-N8 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 31 July 2018
Abbreviations: ER, estrogen receptor [also referred to as HR, hormone receptor]; HER2, human epidermal growth factor receptor-2.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of kinase inhibitors including palbociclib and ribociclib).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents; discusses several tyrosine kinase inhibitors including imatinib, gefitinib, erlotinib and crizotinib, but not palbociclib or ribociclib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/ (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy). - Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016; 6: 740-53. [PubMed: 27217383](Among 225 patients with various solid tumors treated with escalating doses of abemaciclib, the maximum tolerated dose was 200 mg twice daily and highest response rates [31%] were in women with ER+ breast cancer and adverse events were frequent including diarrhea [63%], nausea [45%] and fatigue [41%]; no mention of ALT elevations or hepatotoxicity).
- Dickler MN, Tolaney SM, Rugo HS, Cortés J, Diéras V, Patt D, Wildiers H, et al. MONARCH 1, A phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res 2017; 23: 5218-24. [PMC free article: PMC5581697] [PubMed: 28533223](Among 132 women with refractory, ER+, HER2- metastatic breast cancer treated with abemaciclib [200 mg twice daily] for up to 12 months, the objective response rate was 20% and adverse events were frequent including diarrhea [90%], fatigue [65%], nausea [64%], anorexia [46%] and ALT elevations [30%] which were above 5 times the ULN in 3.8%]).
- Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875-84. [PubMed: 28580882](Among 669 women with refractory, ER+, HER2- metastatic breast cancer treated with fulvestrant in combination with abemaciclib or placebo, progression-free survival was greater with abemaciclib [16 vs 9 months] but adverse events were also greater including diarrhea [86% vs 25%], neutropenia [46% vs 4%], nausea [45% vs 23%], fatigue [40% vs 27%], ALT elevations [41% vs 32%], and ALT levels above 5 times ULN [4.5% vs 1.4%]).
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638-46. [PubMed: 28968163](Among 493 women with HR+, HER2- advanced breast cancer treated with an aromatase inhibitor and either abemaciclib or placebo, the objective response rate was higher with abemaciclib [59% vs 44%] as were adverse events including diarrhea [81% vs 30%], neutropenia [41% vs 2%], fatigue [40% vs 32%], infections [39% vs 29%], ALT elevations [16% vs 15%] and ALT levels above 5 times ULN [6% vs 2%]).
- Kim ES. Abemaciclib: first global approval. Drugs 2017; 77: 2063-70. [PubMed: 29128965](Review of the development, mechanism of action, clinical efficacy and safety of abemaciclib for advanced ER+, HER2- breast cancer).
- Spring LM, Zangardi ML, Moy B, Bardia A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist 2017; 22: 1039-48. [PMC free article: PMC5599204] [PubMed: 28706010](Review of the adverse side effects of palbociclib, ribociclib and abemaciclib and their management, including recommendations for monitoring liver tests and product label recommendations on dose adjustments for liver test abnormalities).
- Abemaciclib (Verzenio)--a third CDK 4/6 inhibitor for breast cancer. Med Lett Drugs Ther 2017; 59 (1533): 185-6. [PubMed: 29125595](Concise review of the mechanism of action, clinical efficacy, toxicity and costs of abemaciclib shortly after its approval for use in the US; mentions that “hepatotoxicity” has been reported with its use).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ribociclib.[LiverTox: Clinical and Researc...]Review Ribociclib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Differences of cyclin-dependent kinase 4/6 inhibitor, palbociclib and abemaciclib, in breast cancer.[Jpn J Clin Oncol. 2019]Review Differences of cyclin-dependent kinase 4/6 inhibitor, palbociclib and abemaciclib, in breast cancer.Tamura K. Jpn J Clin Oncol. 2019 Dec 18; 49(11):993-998.
- Review Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women.[Am J Health Syst Pharm. 2019]Review Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women.Cersosimo RJ. Am J Health Syst Pharm. 2019 Aug 1; 76(16):1183-1202.
- Review Role of cyclin-dependent kinase 4/6 inhibitors in the current and future eras of cancer treatment.[J Oncol Pharm Pract. 2019]Review Role of cyclin-dependent kinase 4/6 inhibitors in the current and future eras of cancer treatment.Parylo S, Vennepureddy A, Dhar V, Patibandla P, Sokoloff A. J Oncol Pharm Pract. 2019 Jan; 25(1):110-129. Epub 2018 May 4.
- Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis.[Cancer Treat Rev. 2020]Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis.Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Cancer Treat Rev. 2020 Nov; 90:102086. Epub 2020 Aug 17.
- Abemaciclib - LiverToxAbemaciclib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...