Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
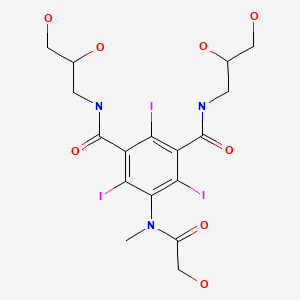
CASRN: 78649-41-9
Drug Levels and Effects
Summary of Use during Lactation
Iomeprol is not approved for marketing in the United States by the U.S. Food and Drug Administration, but is available in other countries. Labeling for the drug in the United Kingdom and guidelines developed by several professional organizations state that breastfeeding need not be disrupted after a nursing mother receives an iodine-containing contrast medium such as iomeprol.[1-4]
Drug Levels
Maternal Levels. Four mothers who were 1 week to 14 months postpartum received iohexol by rapid intravenous injection. Three received a dose of 50 mL (37.8 grams; 17.5 grams of iodine) and one received 60 mL (45.3 grams; 21 grams of iodine). Milk samples of 10 mL were collected 9 times over the 48 hours after the injection. The average iohexol milk concentration over the first 24 hours was 24.6 mg/L in the 3 women 1 week to 4 months postpartum and 130.5 mg/L in the one woman who was 14 months postpartum and weaning her infant. The authors calculated that the average amount of iohexol received by the first 3 infants over the first 24 hours would be 3.7 mg/kg or 0.5% of the weight-adjusted maternal dosage.[5]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
A preterm infant, born at 31 weeks 4 days, was noted at 17 days of age to have a markedly elevated thyrotropin (TSH) value of 87.6 mUI/L (normal range: 0.27 to 4.20 mUI/L). TSH had been normal at birth. Thyroglobulin was also elevated at 811 ng/mL (normal range: 3.5 to 77 ng/mL), but free T4 was normal. The infant had been breastfed (extent not stated) from birth, but also received partial parenteral nutrition until day 10 of life. The infant’s mother had received iomeprol in a dose of 350 mg/kg of iodine at day 4 postpartum and discontinued breastfeeding for 24 hours after the dose. The mother had a history of subclinical hypothyroidism and was treated with levothyroxine.[6] The infant’s transient hypothyroidism, indicated by elevations in TSH and thyroglobulin, was probably caused by the iodine in milk from the contrast medium.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
References
- 1.
- Webb JA, Thomsen HS, Morcos SK, et al. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15:1234–40. [PubMed: 15609057]
- 2.
- Chen MM, Coakley FV, Kaimal A, et al. Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol. 2008;112:333–40. [PubMed: 18669732]
- 3.
- Copel J, El-Sayed Y, Heine RP, et al. Committee Opinion No. 723: Guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2017;130:e210–e216. [PubMed: 28937575]
- 4.
- American College of Radiology Committee on Drugs and Contrast Media. Administration of contrast media to breast-feeding mothers. In, ACR manual on contrast media. 2022;Version 2022:106-7. https://www
.acr.org/Clinical-Resources /Contrast-Manual. - 5.
- Nielsen ST, Matheson I, Rasmussen JN, et al. Excretion of iohexol and metrizoate in human breast milk. Acta Radiol. 1987;28:523–6. [PubMed: 2960342]
- 6.
- Themelin C, Pierron C, Calafat JF, et al. Transient neonatal hypothyroidism secondary to postnatal maternal exposure to contrast medium. BMJ Case Rep. 2019;12:e230854. [PMC free article: PMC6803084] [PubMed: 31619400]
Substance Identification
Substance Name
Iomeprol
CAS Registry Number
78649-41-9
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ioversol.[Drugs and Lactation Database (...]Review Ioversol.. Drugs and Lactation Database (LactMed®). 2006
- Review Iohexol.[Drugs and Lactation Database (...]Review Iohexol.. Drugs and Lactation Database (LactMed®). 2006
- Review Iopromide.[Drugs and Lactation Database (...]Review Iopromide.. Drugs and Lactation Database (LactMed®). 2006
- Review Iodixanol.[Drugs and Lactation Database (...]Review Iodixanol.. Drugs and Lactation Database (LactMed®). 2006
- Review Ioxilan.[Drugs and Lactation Database (...]Review Ioxilan.. Drugs and Lactation Database (LactMed®). 2006
- Iomeprol - Drugs and Lactation Database (LactMed®)Iomeprol - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
