Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
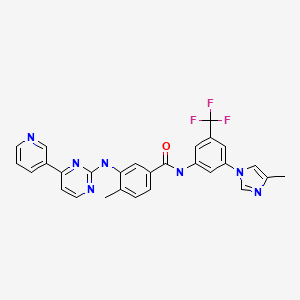
CASRN: 641571-10-0
Drug Levels and Effects
Summary of Use during Lactation
Although the amount of nilotinib in milk appears to be small and one breastfed infant apparently experienced no adverse effects during maternal use of nilotinib, no long-term data are available. Because nilotinib is 98% bound to plasma proteins, the amounts in milk are likely to be low. However, there is little published experience with nilotinib during breastfeeding, and an alternate drug may be preferred, especially while nursing a newborn or preterm infant. National Comprehensive Cancer Network guidelines recommend avoiding breastfeeding during nilotinib therapy and the manufacturer recommends withholding breastfeeding until 2 weeks following the last dose.[1]
Drug Levels
Maternal Levels. One woman with Ph+ chronic myelocytic leukemia was taking nilotinib before pregnancy, but stopped until breastfeeding ceased. She received one dose of nilotinib 400 mg orally and took milk samples at 1, 2, 4, 6, 8, 12 and 24 hours after the dose. She had a peak milk concentration of 129 mcg/L at 4 hours after the dose.[2] These data were incorporated into a physiologically based pharmacokinetic model that predicted the pharmacokinetic profile relatively well. However, more data are needed to validate the model.[3]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
A woman with chronic myeloid leukemia received nilotinib (dosage not stated) for 20 months before pregnancy, throughout pregnancy and continuing during 9 months of breastfeeding (extent not stated). No adverse reactions were reported in her breastfed infant.[4]
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
References
- 1.
- Deininger MW, Shah NP, Altman JK, et al. Chronic myeloid leukemia, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020;18:1385-415. [PubMed: 33022644]
- 2.
- Chelysheva E, Aleshin S, Polushkina E, et al. Breastfeeding in patients with chronic myeloid leukaemia: Case series with measurements of drug concentrations in maternal milk and literature review. Mediterr J Hematol Infect Dis 2018;10:e2018027. [PMC free article: PMC5937977] [PubMed: 29755704]
- 3.
- Liu XI, Leong R, Burckart GJ, Dallmann A. Physiologically-based pharmacokinetic modeling of nilotinib for drug-drug interactions, pediatric patients, and pregnancy and lactation. J Clin Pharmacol 2024;64:323-33. [PubMed: 37909674]
- 4.
- Alizadeh H, Jaafar H, Kajtar B. Outcome of 3 pregnancies in a patient with chronic myeloid leukemia who received 3 types of tyrosine kinase inhibitors each in different pregnancy: Follow-up of the case with a review of published reports. Ann Saudi Med 2015;35:468-71. [PMC free article: PMC6074472] [PubMed: 26657232]
Substance Identification
Substance Name
Nilotinib
CAS Registry Number
641571-10-0
Drug Class
Breast Feeding
Milk, Human
Antineoplastic Agents
Enzyme Inhibitors
Protein Kinase Inhibitors
Signal Transduction Inhibitors
Tyrosine Kinase Inhibitors
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dasatinib.[Drugs and Lactation Database (...]Review Dasatinib.. Drugs and Lactation Database (LactMed®). 2006
- Review Ponatinib.[Drugs and Lactation Database (...]Review Ponatinib.. Drugs and Lactation Database (LactMed®). 2006
- Review Imatinib.[Drugs and Lactation Database (...]Review Imatinib.. Drugs and Lactation Database (LactMed®). 2006
- Review Bosutinib.[Drugs and Lactation Database (...]Review Bosutinib.. Drugs and Lactation Database (LactMed®). 2006
- Review Venetoclax.[Drugs and Lactation Database (...]Review Venetoclax.. Drugs and Lactation Database (LactMed®). 2006
- Nilotinib - Drugs and Lactation Database (LactMed®)Nilotinib - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
