Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Key Messages
- This review identified limited evidence regarding the clinical effectiveness of raltitrexed for patients who had previously experienced adverse events following treatment with fluoropyrimidines. These studies had several limitations, most notably lacking a separate control group; therefore, the effectiveness of raltitrexed in this population is uncertain.
- Limited evidence was found about the safety of raltitrexed for patients who had previously experienced adverse events with fluoropyrimidine treatment. Reported adverse events included cardiac or vascular adverse events, anemia, and nausea and vomiting. No treatment-associated deaths were reported in the studies included in this review.
- No studies were identified that compared the clinical effectiveness of raltitrexed to other therapies, placebo, or no treatment comparator groups for treatment of patients who had experienced adverse events from fluoropyrimidine therapy.
- This review identified evidence for people who had previously experienced severe adverse events, primarily cardiotoxicity, following treatment with fluoropyrimidines. It is unclear if these findings are appliable to patients with a complete dihydropyrimidine dehydrogenase deficiency.
Context and Policy Issues
Cancer has a significant impact on people and health care systems, and is the leading cause of death worldwide and in Canada.1,2 While overall cancer rates, including incidence and mortality have declined, as the population ages and grows in Canada, the number of new cancer cases and deaths is likely to increase. A report from the Canadian Cancer Statistics Advisory Committee estimated that in 2022, there would be 233,900 new cancer cases and 85,100 cancer deaths in Canada.1
Choices for cancer treatment depend on the type of cancer, and options include surgery, radiotherapy, and/or systemic therapies such as chemotherapy or targeted biological therapies.2 Fluoropyrimidines, which include 5-fluorouracil (5-FU) and capecitabine, are commonly used in chemotherapy for multiple types of cancer, including colorectal, breast, head and neck, pancreatic, and gastric.3,4 Fluoropyrimidines are also frequently used with external radiation therapy.3 Worldwide, an estimated 2 million patients are treated with fluoropyrimidines each year.4 However, these drugs are also associated with adverse events, including cardiotoxicities (e.g., angina, myocardial infarction, arrhythmias)3 and non-cardiac adverse events (e.g., mucositis, diarrhea).5 It is estimated that 10% to 40% of patients who are treated with fluoropyrimidines may experience severe toxicities, and that these toxicities may be fatal in approximately 0.5% to 1% of patients.4,6
The risk of cardiotoxicity from fluoropyrimidines may be influenced by numerous factors, including dosing schedule, route of administration, underlying heart disease or other cardiac risk factors, age, type and stage of cancer, and other drugs being used concurrently.3,6 Genetics may also influence the risk of adverse events from fluoropyrimidines. Fluoropyrimidines are metabolized in the body by the dihydropyrimidine dehydrogenase (DPD) enzyme. Genetic variations, such as in the gene that encodes DPD, can result in reduced DPD enzyme activity.3,4 It is estimated that 3% to 8% of the population has a partial DPD deficiency (defined as up to approximately 50% lower activity), and that about 0.1% of the population has a complete DPD deficiency (approximately 0% enzyme activity).4 Studies have estimated that 39% to 61% of patients who experienced severe fluoropyrimidine-associated toxicities had decreased DPD activity.4
DPD deficiency may be assessed through strategies such as genotyping or measuring the DPD phenotype, or may be suspected when a patient experiences cardiotoxicity following treatment with fluoropyrimidine.4 Using a non-fluoropyrimidine treatment may be the preferred strategy, particularly for patients with a complete DPD deficiency.3 An alternative treatment option is raltitrexed, for which Health Canada issued a Notice of Compliance for the treatment of advanced colorectal cancer.7
In September 2022, CADTH published a Reference List on this topic that identified some relevant studies.8 Thus, the purpose of this report is to summarize and critically appraise evidence regarding the clinical effectiveness and safety of raltitrexed in patients with complete DPD deficiency or who are at risk of severe fluoropyrimidine toxicity or intolerance.
Research Questions
- What is the clinical effectiveness of raltitrexed in patients with complete DPD deficiency?
- What is the safety of raltitrexed in patients with complete DPD deficiency?
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources, including MEDLINE, Embase, the Cochrane Database of Systematic Reviews, the International HTA Database, the websites of Canadian and major international health technology agencies, as well as a focused internet search. The search strategy comprised both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were raltitrexed and dihydropyrimidine dehydrogenase deficiency. No filters were applied to limit retrieval by study type. Comments, newspaper articles, editorials, letters, and conference abstracts were excluded. When possible, retrieval was limited to the human population. The search was completed on October 4, 2022, and limited to English-language documents published since January 1, 2012.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in Table 1.
Table 1
Selection Criteria.
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in Table 1, were duplicate publications, or were published before 2012.
Critical Appraisal of Individual Studies
The included publications were critically appraised by 1 reviewer using the following tools as a guide: A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2)9 for the systematic review (SR), and the Downs and Black checklist10 for the non-randomized studies. Summary scores were not calculated for the included studies; rather, the strengths and limitations of each included publication were described narratively.
Summary of Evidence
Quantity of Research Available
A total of 88 citations were identified in the literature search. Following screening of titles and abstracts, 79 citations were excluded and 9 potentially relevant reports from the electronic search were retrieved for full-text review. Of these potentially relevant articles, 4 publications were excluded for various reasons, and 5 publications met the inclusion criteria and were included in this report. These comprised 1 publication that included both an SR and a non-randomized study, and 4 additional non-randomized studies. Appendix 1 presents the PRISMA11 flow chart of the study selection. Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Five publications were included in this report, with 1 publication that included both an SR and a non-randomized study,12 and 4 additional non-randomized studies.13-16 The SR12 and 3 non-randomized studies12,13,15 had broader inclusion criteria than the present review, as they were not restricted to patients who had a DPD deficiency or were at severe risk of fluoropyrimidine toxicity and/or intolerance. Only the characteristics and results of the subset of relevant patients is described in this report.
Additional details regarding the characteristics of the included publications are provided in Appendix 2.
Study Design
One SR12 was identified that was published in 2013 and searched PubMed from January 1, 1991, to August 10, 2011. The authors did not specify if certain study designs were excluded, and encompassed non-randomized studies, including case studies. Their inclusion criteria were broader than this report as they aimed to assess cardiotoxicity following treatment with 5-FU, capecitabine, and raltitrexed. They identified 3 primary studies relevant to this report, which the review authors described as being case studies.
Five non-randomized studies were identified, which were published in 2022,13 2021,13 2018,14 2014,15 and 2013.12 All were retrospective cohort studies: 1 was a population-based review,14 3 were multicentre studies,12,13,16 and 1 was a single-centre study.15
Country of Origin
The first author of the included SR12 was from the UK; the review authors did not report the countries in which the relevant primary studies were conducted.
The non-randomized studies were conducted in Canada,13 Australia,16 France,13 and the UK.12,15
Patient Population
Systematic Review
The SR12 included patients who had experienced cardiotoxicity from treatment with 5-FU or capecitabine, with a separate assessment of studies focused on patients who switched to raltitrexed. The study authors did not report the total number of patients, or patients’ baseline characteristics.
Non-Randomized Studies
For this report, patients who experienced severe adverse side effects from fluoropyrimidine treatment were considered to fit the population inclusion criteria. Two non-randomized studies specifically enrolled patients who had a history of fluoropyrimidine-induced adverse events: 1 included cardiac and non-cardiac adverse events14 and the other included cardiac toxicity only.16 Three non-randomized studies12,13,15 had broader inclusion criteria than this report, and included patients who experienced fluoropyrimidine-induced cardiotoxicity and patients with pre-existing cardiovascular comorbidities. One study13 also included patients with confirmed or suspected DPD deficiency, but did not report outcomes specific to this subgroup.
Two studies13,14 were restricted to patients with metastatic colorectal cancer, 2 studies12,15 focused on patients with gastrointestinal cancer, and 1 study16 included multiple types of cancer (primarily colorectal, but also esophageal and ampullary).
For the patients relevant to this report, the sample size ranged from 42 to 155, and, where reported, the mean or median was between 62 and 66.5 years.13,14,16
Interventions and Comparators
For all studies, the intervention of interest was raltitrexed. Four non-randomized studies13-16 included a mix of single-drug and combination regimens, and 313,14,16 reported the types of combinations. The reported mean or median number of cycles varied across studies.13-16 Three studies13-15 reported the dosages used, with a standard dosage of 3 mg/m2 (2 studies13,14 reported every 3 weeks, 1 study15 did not report the frequency); in some studies, lower dosages13,15 or higher dosages15 were used for some patients. The publication that included both an SR12 and a non-randomized study12 did not report if they included single-drug and/or combination regimens, the mean or median number of cycles, or the dosage.
The comparators relevant to this report were fluoropyrimidines (e.g., before and after with patients who had been previously treated with fluoropyrimidines, or a historical control), or no comparator.
Outcomes
For clinical effectiveness, the reported outcomes were overall survival (OS) and progression-free survival (PFS).13,14
For safety, the reported outcomes were cardiac adverse events (overall12-15 or attributed to raltitrexed16), other adverse events (neutropenia, anemia, nausea and vomiting, diarrhea, and transaminitis),14 and mortality.12,14-16 One study14 also assessed the severity of adverse events retrospectively using the Common Terminology Criteria for Adverse Events, version 5.0.17
Summary of Critical Appraisal
An overview of the critical appraisal of the included studies is summarized in the following section. Additional details regarding the strengths and limitations of the included publications are provided in Appendix 3.
Systematic Review
The identified SR12 stated the aim of the review as well as its population, interventions, and outcomes of interest. Its authors provided their search strategy, and included randomized trials as well as non-randomized studies in their review. They also reported their conflicts of interest and source of financial support.
However, they did not use a comprehensive search strategy, as they did not search multiple databases and or other sources (e.g., grey literature); they also restricted articles to those available in English. Thus, it is possible some relevant studies may have been missed. It was not stated if they had published their methods in advance, or if 2 review authors performed study selection or data extraction. Developing a review protocol in advance and adhering to its methods can help to reduce risk of bias. If study selection and/or data extraction were not conducted in duplicate, there may also be an increased potential for errors. The characteristics of the studies relevant to this report were described in limited detail, which may make it difficult to determine if their findings are potentially applicable to specific groups of patients. In addition, they did not provide a list of excluded studies; thus, it is difficult to determine if potentially relevant studies have been excluded, which may contribute to selection bias. Sources of funding for the included studies were also not reported, so it was also unclear if these results were impacted by their funding source. Quality or risk of bias of the included studies was also not assessed; thus, was not considered during the discussion of the results. Though not formally assessed, the quality of included studies relevant to this review was expected to be low due to the study design (which the review authors described to be case studies).
Non-Randomized Studies
All included non-randomized study authors12-16 clearly described their objective, main outcomes, patient inclusion criteria, and interventions. All were retrospective reviews; thus, it is possible that the patients, treatment, and location may have been representative of typical patients and standard of care. Due to the nature of the intervention, it is expected that compliance with the intervention was reliable. Similarly, as the outcomes were objective, it is expected they were accurate and reliable. The main findings were clearly described, with 4 studies13-16 reporting the 95% confidence interval and/or actual P value for the primary outcomes. All study authors also reported their conflicts of interest.12-16
The 2 studies14,16 that focused on patients who had experienced severe adverse events with fluoropyrimidine treatment described patients’ baseline characteristics. From the 3 studies12,13,15 with broader inclusion criteria, 113 described characteristics of the subgroup of interest to this report, while 2 studies12,15 described their entire sample and not the subgroup of interest. Some studies13-16 reported that patients varied in the number of cycles of raltitrexed they received, but it was unclear if this could have impacted outcomes or if it was considered in the analysis.
As all the included non-randomized studies12-16 were single-arm, retrospective studies, they are at risk of several types of bias. As these studies did not have a separate control group, uncontrolled factors may have affected the findings; thus, these results should be interpreted with caution and may not be attributed entirely to raltitrexed. As patients were not randomized to the intervention, the reported results may have been affected by potential confounding factors. For example, physicians may have switched patients they perceived as being more likely to succeed to raltitrexed; therefore, patients who received raltitrexed in these studies may not have been representative of an average patient. None of the studies included a list of confounders, and it was unclear if confounders were considered in the analysis. One study14 assessed severity of adverse events retrospectively based on electronic medical records; thus, there is a risk of misclassification or underreporting. Specific baseline characteristics and outcomes of interest may also not have been available due to the retrospective design. There was considerable heterogeneity within studies, such as in the type of intervention (e.g., raltitrexed alone versus combination regimens), and it is unclear if this could have influenced the findings.
It was not reported whether the studies that used subgroup analyses12,13,15 were pre-planned. None of the included studies reported blinding the participants or research staff; however, as the outcomes of interest were objective, it is unlikely this introduced bias. Two studies13,16 had fewer than 50 patients relevant to this report; thus, may be statistically underpowered. Three studies12,14,15 also did not clearly report their sources of funding.
Summary of Findings
The main findings from the included SR12 and non-randomized studies12-16 are summarized in this section. Only the findings from the subset of relevant patients are described. Additional details are provided in in Appendix 4.
Clinical Effectiveness of Raltitrexed
Survival
Two single-arm studies13,14 reported on the clinical effectiveness of raltitrexed for patients with metastatic colorectal cancer, as measured by OS and PFS. In one study14 of patients who had experienced cardiac or non-cardiac severe adverse events from fluoropyrimidine treatment, the median OS and PFS were 10.2 months and 8.5 months, respectively. In the other study,13 which had a subgroup of patients who had experienced fluoropyrimidine-induced cardiotoxicity, the median OS and PFS were 28.3 months and 10.6 months, respectively.
One study14 also reported on the survival of patients treated with raltitrexed by different types of cancer. No statistically significant difference was found between colon and rectal cancer. In patients treated with raltitrexed, those with left-sided colon cancer experienced longer median OS and PFS (median 18.9 and 17.1 months, respectively) than patients with right-sided colon cancer (median OS and PFS were both 5.4 months); these differences were statistically significant.
Safety of Raltitrexed
Cardiac and Vascular Adverse Events
All included studies assessed cardiac12-16 or vascular (i.e., cardiovascular or cerebrovascular)12 adverse events following raltitrexed treatment for patients who had previously experienced fluoropyrimidine-related adverse events. The SR12 (which reported findings from 3 case studies or series, from an unclear number of patients) and 2 single-arm studies13,14 reported no cardiac adverse events. One single-arm study16 reported that 1 patient experienced arrhythmia, but stated this was considered to be unrelated to raltitrexed, and that no cardiac events occurred due to raltitrexed. They also stated that this was statistically significantly lower than the expected rate of cardiotoxicity with rechallenging fluoropyrimidines (20%).16 From the remaining 2 single-arm studies, 1 study12 reported that 3 out of 63 patients experienced cardiovascular or cerebrovascular events, and the other15 reported that 8 out of 155 patients (5.2%) experienced cardiac events.
Other Adverse Events
One single-arm study14 reported on other adverse events; anemia was the most common (41.7%), followed by nausea and vomiting (27.4%); neutropenia, diarrhea, and transaminitis were also observed. Most observed adverse events were classified as grade 1 or 2 (mild or moderate); 16.7% of patients experienced grade 3 (severe) adverse events, and no grade 4 (life-threatening) events were observed.14,17
Limitations
The identified SR conducted a limited search, and its methodology may have led to exclusion of relevant studies. They identified 3 case studies or series that were described in limited detail, and did not include a risk of bias assessment. Thus, the quality of evidence identified by this review is unclear, and may be at high risk of bias and uncertainty.
All 5 included primary clinical studies were observational, single-arm retrospective cohort studies. Thus, there is a risk that the findings have been influenced by confounding variables and biases due to their retrospective design, lack of randomization, and lack of a separate control group. Three studies12,13,15 had broader population inclusion criteria than this review, and reported limited information regarding baseline characteristics and outcomes specific to the patients relevant for this report. There was also clinical heterogeneity within and across studies regarding patients’ health status, type of cancer, and treatment protocols, which may have impacted the findings. For example, 1 study14 conducted a multivariable regression analysis to assess factors that may be associated with survival outcomes, and reported that left-sided colon cancer and a baseline ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1 (a measure of a patient’s daily living abilities)18 were associated with longer PFS. These differences may also impact the generalizability of the reported findings to specific patient groups.
For the studies that reported survival outcomes,13,14 there was no comparison to a separate control group, so it is unclear how clinically effective raltitrexed is compared to other treatments available to patients. Relevant studies that assessed the impact of raltitrexed on other measures of clinical effectiveness (e.g., quality of life) were also not identified. The non-randomized studies with broader inclusion criteria12,13,15 reported some outcomes for their full sample but not for the subgroup of interest to this report; as these results included a mixed population, they were not incorporated in this review.
The study findings were specific to patients who had experienced adverse events following treatment with fluoropyrimidines, and it was unclear if these patients had a DPD deficiency. Thus, it is unclear if these findings are generalizable to patients with a complete DPD deficiency.
Where reported, baseline age data also indicates pediatric patients were likely not included in the included studies; thus, the effectiveness and safety of raltitrexed for pediatric patients is unclear.
The generalizability of these findings is also unclear. All studies included data from 2008 or earlier, and it is unknown if older results are applicable to today (e.g., if the quality of treatment has improved). One study14 was conducted in Canada (Alberta), but it is unclear if the findings from the other studies are applicable to the Canadian context.
Conclusions and Implications for Decision- or Policy-Making
This report included 1 SR12 and 5 non-randomized studies12-16 related to the clinical effectiveness and/or safety of raltitrexed for patients who had previously been treated with fluoropyrimidines and experienced adverse events.
Two non-randomized studies13,14 reported on the clinical effectiveness of raltitrexed. One study14 reported that the median OS and PFS were 10.2 months and 8.5 months, respectively, while the other study13 reported a median OS and PFS of 28.3 months and 10.6 months, respectively. As they did not have a relevant control group, it is unclear how clinically effective raltitrexed is compared to alternative treatments. Subgroup analyses in patients treated with raltitrexed indicated that patients with left-sided colon cancer experienced statistically significantly longer median OS and PFS than patients with right-sided colon cancer, and no significant difference was found between colon and rectal cancer.
The SR12 and 5 non-randomized studies12-16 reported outcomes related to safety, and stated that the proportion of patients who experienced cardiac (or cardiovascular or cerebrovascular) adverse events following treatment with raltitrexed ranged between 0% and 5.2%. One non-randomized study14 also reported other types of adverse events: the most common was anemia (41.7%), followed by nausea and vomiting (27.4%), and most adverse events were grade 1 or 2 in severity on the Common Terminology Criteria for Adverse Events scale. Among the studies that reported mortality, 3 studies12,14,15 reported no treatment-related deaths, and 1 study16 reported 1 death considered to be unrelated to raltitrexed.
The limitations of the included publications should be considered when interpreting the findings of this report. Overall, few studies were identified, and comprised 1 SR based on case reports12 and 5 retrospective, single-arm non-randomized studies.12-16 The methodological limitations and heterogeneity within and across studies make it difficult to draw definitive conclusions regarding the effectiveness of raltitrexed for patients who have experienced adverse events with fluoropyrimidine treatment, as the results may have been influenced by various sources of bias and uncertainty. Three non-randomized studies12,13,15 had broader inclusion criteria than this review and did not report all outcomes for the subgroup of interest; thus, these outcomes were not included in this review. It is also unclear if these findings are generalizable to patients with a complete DPD deficiency. It is possible that some or all of the patients from the included studies may have experienced adverse events from fluoropyrimidine treatment for alternate reasons, and it is unclear if these patients may respond differently to raltitrexed compared to patients with a DPD deficiency.
Future high-quality studies that evaluate raltitrexed compared to other treatments (e.g., standard of care) or placebo control groups for patients with a complete DPD deficiency, or for patients who are known to have a high risk of fluoropyrimidine toxicity and/or intolerance, would assist stakeholders with decision-making regarding the use of raltitrexed for these patient groups. Future studies may also consider assessing what variables are associated with greater clinical effectiveness of raltitrexed (e.g., type of cancer) to improve treatment selection. Reporting on other outcomes, such as response rate, quality of life, and other non-cardiac adverse events, may also help further develop an understanding of raltitrexed’s clinical effectiveness and safety.
Abbreviations
- 5-FU
fluorouracil
- AMSTAR 2
A MeaSurement Tool to Assess systematic Reviews 2
- DPD
dihydropyrimidine dehydrogenase
- ECOG
Eastern Cooperative Oncology Group
- OS
overall survival
- PFS
progression-free survival
- SR
systematic review
References
- 1.
- Brenner DR, Poirier A, Woods RR, et al. Projected estimates of cancer in Canada in 2022. Can Med Assoc J. 2022;194(17):E601-E607. [PMC free article: PMC9067380] [PubMed: 35500919]
- 2.
- Cancer. Geneva (CH): World Health Organization; 2022: https://www
.who.int/news-room /fact-sheets/detail/cancer. Accessed 2022 Oct 6. - 3.
- Saif WM, Banchs J, Kohne C-H. Fluoropyrimidine-associated cardiotoxicity: incidence, clinical manifestations, mechanisms, and management. In: Post TW, ed. UpToDate. Waltham (MA): UpToDate; 2022: https://www
.uptodate.com. Accessed 2022 Oct 20. - 4.
- Henricks LM, Opdam FL, Beijnen JH, Cats A, Schellens JHM. DPYD genotype-guided dose individualization to improve patient safety of fluoropyrimidine therapy: call for a drug label update. Ann Oncol. 2017;28(12):2915-2922. [PubMed: 29045513]
- 5.
- Padegimas A, Carver JR. How to diagnose and manage patients with fluoropyrimidine-induced chest pain. JACC CardioOncol. 2020;2(4):650-654. [PMC free article: PMC8352252] [PubMed: 34396276]
- 6.
- DPYD genotyping in patients who have planned cancer treatment with fluoropyrimidines: a health technology assessment. Ont Health Technol Assess Ser. 2021;21(14):1-186. [PMC free article: PMC8382304] [PubMed: 34484488]
- 7.
- Tomudex® (raltitrexed): lyophilized powder, 2 mg raltitrexed per vial, intravenous injection [product monograph]. Kirkland (QC): Pfizer Canada; 2021 Dec 7: https://pdf
.hres.ca/dpd_pm/00063865.PDF. Accessed 2022 Oct 31. - 8.
- Madakadze C, Premji Z, Bailey S. Raltitrexed in patients with dihydropyrimidine dehydrogenase (DPD) deficiency. (CADTH reference list). Ottawa (ON): CADTH; 2022: https://www
.cadth.ca /raltitrexed-patients-dihydropyrimidine-dehydrogenase-dpd-deficiency. Accessed 2022 Oct 20. - 9.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [PMC free article: PMC5833365] [PubMed: 28935701]
- 10.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [PMC free article: PMC1756728] [PubMed: 9764259]
- 11.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. [PubMed: 19631507]
- 12.
- Kelly C, Bhuva N, Harrison M, Buckley A, Saunders M. Use of raltitrexed as an alternative to 5-fluorouracil and capecitabine in cancer patients with cardiac history. Eur J Cancer. 2013;49(10):2303-2310. [PubMed: 23583220]
- 13.
- Gallois C, Hafliger E, Auclin E, et al. First-line chemotherapy with raltitrexed in metastatic colorectal cancer: an Association des Gastro-enterologues Oncologues (AGEO) multicentre study. Dig Liver Dis. 2022;54(5):684-691. [PubMed: 34470724]
- 14.
- Batra A, Rigo R, Hannouf MB, Cheung WY. Real-world safety and efficacy of raltitrexed in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2021;20(2):e75-e81. [PubMed: 33268287]
- 15.
- Khan K, Rane JK, Cunningham D, et al. Efficacy and cardiotoxic safety profile of raltitrexed in fluoropyrimidines-pretreated or high-risk cardiac patients with GI malignancies: large single-center experience. Clin Colorectal Cancer. 2019;18(1):64-71.e61. [PubMed: 30404764]
- 16.
- Ransom D, Wilson K, Fournier M, et al. Final results of Australasian Gastrointestinal Trials Group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol. 2014;25(1):117-121. [PubMed: 24299960]
- 17.
- Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Bethesda (MD): U.S. Department of Health and Human Sciences; 2017: https://ctep
.cancer.gov /protocoldevelopment /electronic_applications /docs/CTCAE_v5_Quick_Reference_8 .5x11.pdf. Accessed 2022 Oct 21. - 18.
- ECOG Performance Status Scale. Philadelphia (PA): ECOG-ACRIN cancer research group; 2022: https://ecog-acrin
.org /resources/ecog-performance-status/. Accessed 2022 Oct 19.
Appendix 1. Selection of Included Studies
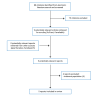
Figure 1
Selection of Included Studies.
Appendix 2. Characteristics of Included Publications
Note that this appendix has not been copy-edited.
Table 2
Characteristics of Included Systematic Review.
Table 3
Characteristics of Included Non-Randomized Studies.
Appendix 3. Critical Appraisal of Included Publications
Note that this appendix has not been copy-edited.
Table 4
Strengths and Limitations of Systematic Review Using AMSTAR 29.
Table 5
Strengths and Limitations of Clinical Studies Using the Downs and Black Checklist.
Appendix 4. Main Study Findings
Note that this appendix has not been copy-edited.
Table 6
Summary of Findings by Outcome — Survival.
Table 7
Summary of Findings by Outcome — Cardiac and Vascular Adverse Events.
Table 8
Summary of Findings by Outcome — Other Adverse Events.
Table 9
Summary of Findings by Outcome — Mortality.
Appendix 5. References of Potential Interest
- Madakadze C, Premji Z, Bailey S. Raltitrexed in patients with dihydropyrimidine dehydrogenase (DPD) deficiency. (CADTH reference list). Ottawa (ON): CADTH; 2022: https://www.cadth.ca/raltitrexed-patients-dihydropyrimidine-dehydrogenase-dpd-deficiency. Accessed 2022 Oct 5.
- Li X, Shen J, Xia F, Zhu J. Efficacy and safety of radiotherapy combined with raltitrexed and irinotecan for treating unresectable recurrent colorectal cancer: a single-arm phase II trial. J Gastrointest Oncol. 2022 Jun;13(3):1112-1120. [PMC free article: PMC9274057] [PubMed: 35837190]
- Cucciniello L, Bidoli E, Viel E, Canale ML, Gerratana L, Lestuzzi C. The puzzling clinical presentation of fluoropyrimidines cardiotoxicity. Front. 2022;9:960240. [PMC free article: PMC9515374] [PubMed: 36186986]
- Winquist LE, Sanatani M, Kim RB, Winquist E. Near miss or standard of care? DPYD screening for cancer patients receiving fluorouracil. Curr Oncol. 2021 February;28(1):94-97. [PMC free article: PMC7816174] [PubMed: 33704179]
Previous CADTH Reports
Non-Randomized Studies
Mixed Population – Patients Refractory or Intolerant to Fluoropyrimidine
Case Series and Reports
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up to date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Questions or requests for information about this report can be directed to Requests@CADTH.ca.
- Key Messages
- Context and Policy Issues
- Research Questions
- Methods
- Summary of Evidence
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- Selection of Included Studies
- Characteristics of Included Publications
- Critical Appraisal of Included Publications
- Main Study Findings
- References of Potential Interest
- Raltitrexed in Patients With Dihydropyrimidine Dehydrogenase DeficiencyRaltitrexed in Patients With Dihydropyrimidine Dehydrogenase Deficiency
Your browsing activity is empty.
Activity recording is turned off.
See more...
