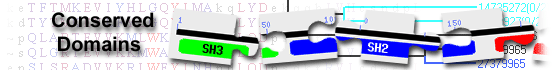ribitol 5-phosphate transferase FKRP isoform X1 [Homo sapiens]
List of domain hits

| Name | Accession | Description | Interval | E-value | |||
| LicD super family | cl01378 | LicD family; The LICD family of proteins show high sequence similarity and are involved in ... |
319-371 | 1.83e-13 | |||
LicD family; The LICD family of proteins show high sequence similarity and are involved in phosphorylcholine metabolism. There is evidence to show that LicD2 mutants have a reduced ability to take up choline, have decreased ability to adhere to host cells and are less virulent. These proteins are part of the nucleotidyltransferase superfamily. The actual alignment was detected with superfamily member COG3475: Pssm-ID: 470175 Cd Length: 266 Bit Score: 70.39 E-value: 1.83e-13
|
|||||||
| ispDF super family | cl35819 | bifunctional 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-D-erythritol ... |
67-161 | 6.30e-03 | |||
bifunctional 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase protein; Provisional The actual alignment was detected with superfamily member PRK09382: Pssm-ID: 236492 [Multi-domain] Cd Length: 378 Bit Score: 38.68 E-value: 6.30e-03
|
|||||||
| Name | Accession | Description | Interval | E-value | |||
| LicD | COG3475 | Phosphorylcholine metabolism protein LicD [Lipid transport and metabolism]; |
319-371 | 1.83e-13 | |||
Phosphorylcholine metabolism protein LicD [Lipid transport and metabolism]; Pssm-ID: 442698 Cd Length: 266 Bit Score: 70.39 E-value: 1.83e-13
|
|||||||
| LicD | pfam04991 | LicD family; The LICD family of proteins show high sequence similarity and are involved in ... |
334-371 | 2.32e-10 | |||
LicD family; The LICD family of proteins show high sequence similarity and are involved in phosphorylcholine metabolism. There is evidence to show that LicD2 mutants have a reduced ability to take up choline, have decreased ability to adhere to host cells and are less virulent. These proteins are part of the nucleotidyltransferase superfamily. Pssm-ID: 428243 Cd Length: 228 Bit Score: 60.47 E-value: 2.32e-10
|
|||||||
| ispDF | PRK09382 | bifunctional 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-D-erythritol ... |
67-161 | 6.30e-03 | |||
bifunctional 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase protein; Provisional Pssm-ID: 236492 [Multi-domain] Cd Length: 378 Bit Score: 38.68 E-value: 6.30e-03
|
|||||||
| Name | Accession | Description | Interval | E-value | |||
| LicD | COG3475 | Phosphorylcholine metabolism protein LicD [Lipid transport and metabolism]; |
319-371 | 1.83e-13 | |||
Phosphorylcholine metabolism protein LicD [Lipid transport and metabolism]; Pssm-ID: 442698 Cd Length: 266 Bit Score: 70.39 E-value: 1.83e-13
|
|||||||
| LicD | pfam04991 | LicD family; The LICD family of proteins show high sequence similarity and are involved in ... |
334-371 | 2.32e-10 | |||
LicD family; The LICD family of proteins show high sequence similarity and are involved in phosphorylcholine metabolism. There is evidence to show that LicD2 mutants have a reduced ability to take up choline, have decreased ability to adhere to host cells and are less virulent. These proteins are part of the nucleotidyltransferase superfamily. Pssm-ID: 428243 Cd Length: 228 Bit Score: 60.47 E-value: 2.32e-10
|
|||||||
| ispDF | PRK09382 | bifunctional 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-D-erythritol ... |
67-161 | 6.30e-03 | |||
bifunctional 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase/2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase protein; Provisional Pssm-ID: 236492 [Multi-domain] Cd Length: 378 Bit Score: 38.68 E-value: 6.30e-03
|
|||||||
| Blast search parameters | ||||
|