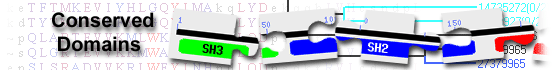bcl2-associated agonist of cell death [Homo sapiens]
Bcl-2_BAD domain-containing protein( domain architecture ID 10565048)
Bcl-2_BAD domain-containing protein
List of domain hits

| Name | Accession | Description | Interval | E-value | ||||
| Bcl-2_BAD | pfam10514 | Pro-apoptotic Bcl-2 protein, BAD; BAD is a Bcl-2 homology domain 3 (BH3)-only pro-apoptotic ... |
1-168 | 5.82e-92 | ||||
Pro-apoptotic Bcl-2 protein, BAD; BAD is a Bcl-2 homology domain 3 (BH3)-only pro-apoptotic member of the Bcl-2 protein family that is regulated by phosphorylation in response to survival factors. Binding of BAD to mitochondria is thought to be exclusively mediated by its BH3 domain. Membrane localization of BAD mediates membrane translocation of Bcl-XL. The C-terminal part of BAD is sufficient for membrane binding. There are two segments with differing lipid-binding preferences, LBD1 and LBD2, that are responsible for this binding: (i) LBD1 located in the proximity of the BH3 domain (amino acids 122-131) and (ii) LBD2, the putative C-terminal alpha-helix-5. Phosphorylation-regulated 14-3-3 protein binding may expose the cholesterol-preferring LBD1 and bury the LBD2, thereby mediating translocation of BAD to raft-like micro-domains. : Pssm-ID: 402236 Cd Length: 166 Bit Score: 264.56 E-value: 5.82e-92
|
||||||||
| Name | Accession | Description | Interval | E-value | ||||
| Bcl-2_BAD | pfam10514 | Pro-apoptotic Bcl-2 protein, BAD; BAD is a Bcl-2 homology domain 3 (BH3)-only pro-apoptotic ... |
1-168 | 5.82e-92 | ||||
Pro-apoptotic Bcl-2 protein, BAD; BAD is a Bcl-2 homology domain 3 (BH3)-only pro-apoptotic member of the Bcl-2 protein family that is regulated by phosphorylation in response to survival factors. Binding of BAD to mitochondria is thought to be exclusively mediated by its BH3 domain. Membrane localization of BAD mediates membrane translocation of Bcl-XL. The C-terminal part of BAD is sufficient for membrane binding. There are two segments with differing lipid-binding preferences, LBD1 and LBD2, that are responsible for this binding: (i) LBD1 located in the proximity of the BH3 domain (amino acids 122-131) and (ii) LBD2, the putative C-terminal alpha-helix-5. Phosphorylation-regulated 14-3-3 protein binding may expose the cholesterol-preferring LBD1 and bury the LBD2, thereby mediating translocation of BAD to raft-like micro-domains. Pssm-ID: 402236 Cd Length: 166 Bit Score: 264.56 E-value: 5.82e-92
|
||||||||
| Name | Accession | Description | Interval | E-value | ||||
| Bcl-2_BAD | pfam10514 | Pro-apoptotic Bcl-2 protein, BAD; BAD is a Bcl-2 homology domain 3 (BH3)-only pro-apoptotic ... |
1-168 | 5.82e-92 | ||||
Pro-apoptotic Bcl-2 protein, BAD; BAD is a Bcl-2 homology domain 3 (BH3)-only pro-apoptotic member of the Bcl-2 protein family that is regulated by phosphorylation in response to survival factors. Binding of BAD to mitochondria is thought to be exclusively mediated by its BH3 domain. Membrane localization of BAD mediates membrane translocation of Bcl-XL. The C-terminal part of BAD is sufficient for membrane binding. There are two segments with differing lipid-binding preferences, LBD1 and LBD2, that are responsible for this binding: (i) LBD1 located in the proximity of the BH3 domain (amino acids 122-131) and (ii) LBD2, the putative C-terminal alpha-helix-5. Phosphorylation-regulated 14-3-3 protein binding may expose the cholesterol-preferring LBD1 and bury the LBD2, thereby mediating translocation of BAD to raft-like micro-domains. Pssm-ID: 402236 Cd Length: 166 Bit Score: 264.56 E-value: 5.82e-92
|
||||||||
| Blast search parameters | ||||
|