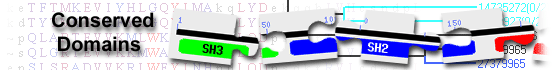mitotic checkpoint serine/threonine-protein kinase BUB1 beta [Homo sapiens]
List of domain hits

| Name | Accession | Description | Interval | E-value | |||||
| STKc_BubR1_vert | cd14029 | Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint ... |
750-1045 | 0e+00 | |||||
Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint protein BubR1; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. BubR1 (Budding uninhibited by benzimidazoles R1) is also called Bub1 beta (Bub1b). It contains an N-terminal Bub1/Mad3 homology domain essential for Cdc20 binding and a C-terminal kinase domain. It is involved in SAC, a surveillance system that delays metaphase to anaphase transition by blocking the activity of APC/C (the anaphase promoting complex) until all chromosomes achieve proper attachments to the mitotic spindle, to avoid chromosome missegregation. BubR1 inhibits APC/C through direct binding. It also plays an important role in stabilizing kinetochore-microtubule attachments. Mutant mice expressing only 10% normal BubR1 protein are viable and develop into adult mice, but display many early aging-associated phenotypes including reduced lifespan, muscle atrophy, cataracts, impaired wound healing, and infertility. The BubR1 subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. : Pssm-ID: 270931 [Multi-domain] Cd Length: 304 Bit Score: 550.24 E-value: 0e+00
|
|||||||||
| Mad3_BUB1_I | pfam08311 | Mad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved ... |
57-179 | 3.64e-57 | |||||
Mad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved in cell division. This region has been shown to be essential for the binding of the binding of BUB1 and MAD3 to CDC20p. : Pssm-ID: 462420 Cd Length: 123 Bit Score: 192.74 E-value: 3.64e-57
|
|||||||||
| PTZ00121 super family | cl31754 | MAEBL; Provisional |
369-488 | 2.91e-04 | |||||
MAEBL; Provisional The actual alignment was detected with superfamily member PTZ00121: Pssm-ID: 173412 [Multi-domain] Cd Length: 2084 Bit Score: 45.13 E-value: 2.91e-04
|
|||||||||
| Name | Accession | Description | Interval | E-value | |||||
| STKc_BubR1_vert | cd14029 | Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint ... |
750-1045 | 0e+00 | |||||
Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint protein BubR1; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. BubR1 (Budding uninhibited by benzimidazoles R1) is also called Bub1 beta (Bub1b). It contains an N-terminal Bub1/Mad3 homology domain essential for Cdc20 binding and a C-terminal kinase domain. It is involved in SAC, a surveillance system that delays metaphase to anaphase transition by blocking the activity of APC/C (the anaphase promoting complex) until all chromosomes achieve proper attachments to the mitotic spindle, to avoid chromosome missegregation. BubR1 inhibits APC/C through direct binding. It also plays an important role in stabilizing kinetochore-microtubule attachments. Mutant mice expressing only 10% normal BubR1 protein are viable and develop into adult mice, but display many early aging-associated phenotypes including reduced lifespan, muscle atrophy, cataracts, impaired wound healing, and infertility. The BubR1 subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 270931 [Multi-domain] Cd Length: 304 Bit Score: 550.24 E-value: 0e+00
|
|||||||||
| Mad3_BUB1_I | pfam08311 | Mad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved ... |
57-179 | 3.64e-57 | |||||
Mad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved in cell division. This region has been shown to be essential for the binding of the binding of BUB1 and MAD3 to CDC20p. Pssm-ID: 462420 Cd Length: 123 Bit Score: 192.74 E-value: 3.64e-57
|
|||||||||
| Mad3_BUB1_I | smart00777 | Mad3/BUB1 hoMad3/BUB1 homology region 1; Proteins containing this domain are checkpoint ... |
56-178 | 7.18e-56 | |||||
Mad3/BUB1 hoMad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved in cell division. This region has been shown to be essential for the binding of the binding of BUB1 and MAD3 to CDC20p. Pssm-ID: 214817 Cd Length: 124 Bit Score: 188.97 E-value: 7.18e-56
|
|||||||||
| PRK14879 | PRK14879 | Kae1-associated kinase Bud32; |
837-920 | 3.98e-05 | |||||
Kae1-associated kinase Bud32; Pssm-ID: 237847 [Multi-domain] Cd Length: 211 Bit Score: 46.05 E-value: 3.98e-05
|
|||||||||
| arch_bud32 | TIGR03724 | Kae1-associated kinase Bud32; Members of this protein family are the Bud32 protein associated ... |
837-920 | 1.04e-04 | |||||
Kae1-associated kinase Bud32; Members of this protein family are the Bud32 protein associated with Kae1 (kinase-associated endopeptidase 1) in the Archaea. In many Archaeal genomes, Kae1 and Bud32 are fused. The complex is homologous to the Kae1 and Bud32 subunits of the eukaryotic KEOPS complex, an apparently ancient protein kinase-containing molecular machine. [Unknown function, General] Pssm-ID: 274749 [Multi-domain] Cd Length: 199 Bit Score: 44.51 E-value: 1.04e-04
|
|||||||||
| PTZ00121 | PTZ00121 | MAEBL; Provisional |
369-488 | 2.91e-04 | |||||
MAEBL; Provisional Pssm-ID: 173412 [Multi-domain] Cd Length: 2084 Bit Score: 45.13 E-value: 2.91e-04
|
|||||||||
| DUF4670 | pfam15709 | Domain of unknown function (DUF4670); This family of proteins is found in eukaryotes. Proteins ... |
373-492 | 2.57e-03 | |||||
Domain of unknown function (DUF4670); This family of proteins is found in eukaryotes. Proteins in this family are typically between 373 and 763 amino acids in length. Pssm-ID: 464815 [Multi-domain] Cd Length: 522 Bit Score: 41.48 E-value: 2.57e-03
|
|||||||||
| Bud32 | COG3642 | tRNA A-37 threonylcarbamoyl transferase component Bud32 [Translation, ribosomal structure and ... |
826-920 | 8.30e-03 | |||||
tRNA A-37 threonylcarbamoyl transferase component Bud32 [Translation, ribosomal structure and biogenesis]; tRNA A-37 threonylcarbamoyl transferase component Bud32 is part of the Pathway/BioSystem: tRNA modification Pssm-ID: 442859 [Multi-domain] Cd Length: 159 Bit Score: 38.02 E-value: 8.30e-03
|
|||||||||
| Name | Accession | Description | Interval | E-value | |||||
| STKc_BubR1_vert | cd14029 | Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint ... |
750-1045 | 0e+00 | |||||
Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint protein BubR1; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. BubR1 (Budding uninhibited by benzimidazoles R1) is also called Bub1 beta (Bub1b). It contains an N-terminal Bub1/Mad3 homology domain essential for Cdc20 binding and a C-terminal kinase domain. It is involved in SAC, a surveillance system that delays metaphase to anaphase transition by blocking the activity of APC/C (the anaphase promoting complex) until all chromosomes achieve proper attachments to the mitotic spindle, to avoid chromosome missegregation. BubR1 inhibits APC/C through direct binding. It also plays an important role in stabilizing kinetochore-microtubule attachments. Mutant mice expressing only 10% normal BubR1 protein are viable and develop into adult mice, but display many early aging-associated phenotypes including reduced lifespan, muscle atrophy, cataracts, impaired wound healing, and infertility. The BubR1 subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 270931 [Multi-domain] Cd Length: 304 Bit Score: 550.24 E-value: 0e+00
|
|||||||||
| Mad3_BUB1_I | pfam08311 | Mad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved ... |
57-179 | 3.64e-57 | |||||
Mad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved in cell division. This region has been shown to be essential for the binding of the binding of BUB1 and MAD3 to CDC20p. Pssm-ID: 462420 Cd Length: 123 Bit Score: 192.74 E-value: 3.64e-57
|
|||||||||
| Mad3_BUB1_I | smart00777 | Mad3/BUB1 hoMad3/BUB1 homology region 1; Proteins containing this domain are checkpoint ... |
56-178 | 7.18e-56 | |||||
Mad3/BUB1 hoMad3/BUB1 homology region 1; Proteins containing this domain are checkpoint proteins involved in cell division. This region has been shown to be essential for the binding of the binding of BUB1 and MAD3 to CDC20p. Pssm-ID: 214817 Cd Length: 124 Bit Score: 188.97 E-value: 7.18e-56
|
|||||||||
| STKc_Bub1_BubR1 | cd13981 | Catalytic domain of the Serine/Threonine kinases, Spindle assembly checkpoint proteins Bub1 ... |
752-1043 | 4.41e-45 | |||||
Catalytic domain of the Serine/Threonine kinases, Spindle assembly checkpoint proteins Bub1 and BubR1; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. This subfamily is composed of Bub1 (Budding uninhibited by benzimidazoles 1), BubR1, and similar proteins. They contain an N-terminal Bub1/Mad3 homology domain essential for Cdc20 binding and a C-terminal kinase domain. Bub1 and BubR1 are involved in SAC, a surveillance system that delays metaphase to anaphase transition by blocking the activity of APC/C (the anaphase promoting complex) until all chromosomes achieve proper attachments to the mitotic spindle, to avoid chromosome missegregation. Impaired SAC leads to genomic instabilities and tumor development. Bub1 and BubR1 facilitate the localization of SAC proteins to kinetochores and regulate kinetochore-microtubule (K-MT) attachments. Repression studies of Bub1 and BubR1 show that they exert an additive effect in misalignment phenotypes and may function cooperatively or in parallel pathways in regulating K-MT attachments. The Bub1/BubR1 subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 270883 [Multi-domain] Cd Length: 298 Bit Score: 164.84 E-value: 4.41e-45
|
|||||||||
| STKc_Bub1_vert | cd14028 | Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint ... |
785-1031 | 2.56e-27 | |||||
Catalytic domain of the Serine/Threonine kinase, Vertebrate Spindle assembly checkpoint protein Bub1; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. Bub1 (Budding uninhibited by benzimidazoles 1) contains an N-terminal Bub1/Mad3 homology domain essential for Cdc20 binding, a GLEBS motif for Bub3/kinetochore binding, and a C-terminal kinase domain. It is involved in SAC, a surveillance system that delays metaphase to anaphase transition by blocking the activity of APC/C (the anaphase promoting complex) until all chromosomes achieve proper attachments to the mitotic spindle, to avoid chromosome missegregation. Bub1 contributes to the inhibition of APC/C by phosphorylating its crucial cofactor, Cdc20, rendering it unable to activate APC/C. In addition, Bub1 facilitates the localization to kinetochores of other SAC and motor proteins including Mad1, Mad2, BubR1, and Plk1. It acts as the master organizer of the functional inner centromere. Bub1 also play roles in protecting sister chromatid cohesion and normal metaphase congression. The Bub1 subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 270930 [Multi-domain] Cd Length: 290 Bit Score: 113.02 E-value: 2.56e-27
|
|||||||||
| PKc | cd00180 | Catalytic domain of Protein Kinases; PKs catalyze the transfer of the gamma-phosphoryl group ... |
793-963 | 4.56e-06 | |||||
Catalytic domain of Protein Kinases; PKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine or tyrosine residues on protein substrates. PKs make up a large family of serine/threonine kinases (STKs), protein tyrosine kinases (PTKs), and dual-specificity PKs that phosphorylate both serine/threonine and tyrosine residues of target proteins. Majority of protein phosphorylation occurs on serine residues while only 1% occurs on tyrosine residues. Protein phosphorylation is a mechanism by which a wide variety of cellular proteins, such as enzymes and membrane channels, are reversibly regulated in response to certain stimuli. PKs often function as components of signal transduction pathways in which one kinase activates a second kinase, which in turn, may act on other kinases; this sequential action transmits a signal from the cell surface to target proteins, which results in cellular responses. The PK family is one of the largest known protein families with more than 100 homologous yeast enzymes and more than 500 human proteins. A fraction of PK family members are pseudokinases that lack crucial residues for catalytic activity. The mutiplicity of kinases allows for specific regulation according to substrate, tissue distribution, and cellular localization. PKs regulate many cellular processes including proliferation, division, differentiation, motility, survival, metabolism, cell-cycle progression, cytoskeletal rearrangement, immunity, and neuronal functions. Many kinases are implicated in the development of various human diseases including different types of cancer. The PK family is part of a larger superfamily that includes the catalytic domains of RIO kinases, aminoglycoside phosphotransferase, choline kinase, phosphoinositide 3-kinase (PI3K), and actin-fragmin kinase. Pssm-ID: 270622 [Multi-domain] Cd Length: 215 Bit Score: 48.81 E-value: 4.56e-06
|
|||||||||
| PRK14879 | PRK14879 | Kae1-associated kinase Bud32; |
837-920 | 3.98e-05 | |||||
Kae1-associated kinase Bud32; Pssm-ID: 237847 [Multi-domain] Cd Length: 211 Bit Score: 46.05 E-value: 3.98e-05
|
|||||||||
| arch_bud32 | TIGR03724 | Kae1-associated kinase Bud32; Members of this protein family are the Bud32 protein associated ... |
837-920 | 1.04e-04 | |||||
Kae1-associated kinase Bud32; Members of this protein family are the Bud32 protein associated with Kae1 (kinase-associated endopeptidase 1) in the Archaea. In many Archaeal genomes, Kae1 and Bud32 are fused. The complex is homologous to the Kae1 and Bud32 subunits of the eukaryotic KEOPS complex, an apparently ancient protein kinase-containing molecular machine. [Unknown function, General] Pssm-ID: 274749 [Multi-domain] Cd Length: 199 Bit Score: 44.51 E-value: 1.04e-04
|
|||||||||
| STKc_PhKG1 | cd14182 | Catalytic domain of the Serine/Threonine Kinase, Phosphorylase kinase Gamma 1 subunit; STKs ... |
842-948 | 2.12e-04 | |||||
Catalytic domain of the Serine/Threonine Kinase, Phosphorylase kinase Gamma 1 subunit; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. Phosphorylase kinase (PhK) catalyzes the phosphorylation of inactive phosphorylase b to form the active phosphorylase a. It coordinates hormonal, metabolic, and neuronal signals to initiate the breakdown of glycogen stores, which enables the maintenance of blood-glucose homeostasis during fasting, and is also used as a source of energy for muscle contraction. PhK is one of the largest and most complex protein kinases, composed of a heterotetramer containing four molecules each of four subunit types: one catalytic (gamma) and three regulatory (alpha, beta, and delta). The gamma 1 subunit (PhKG1) is also referred to as the muscle gamma isoform. The gamma subunit, when isolated, is constitutively active and does not require phosphorylation of the A-loop for activity. The regulatory subunits restrain this kinase activity until signals are received to relieve this inhibition. For example, the kinase is activated in response to hormonal stimulation, after autophosphorylation or phosphorylation by cAMP-dependent kinase of the alpha and beta subunits. The high-affinity binding of ADP to the beta subunit also stimulates kinase activity, whereas calcium relieves inhibition by binding to the delta (calmodulin) subunit. The PhKG1 subfamily is part of a larger superfamily that includes the catalytic domains of other STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 271084 [Multi-domain] Cd Length: 276 Bit Score: 44.52 E-value: 2.12e-04
|
|||||||||
| PTZ00121 | PTZ00121 | MAEBL; Provisional |
369-488 | 2.91e-04 | |||||
MAEBL; Provisional Pssm-ID: 173412 [Multi-domain] Cd Length: 2084 Bit Score: 45.13 E-value: 2.91e-04
|
|||||||||
| PTZ00121 | PTZ00121 | MAEBL; Provisional |
373-488 | 6.89e-04 | |||||
MAEBL; Provisional Pssm-ID: 173412 [Multi-domain] Cd Length: 2084 Bit Score: 43.98 E-value: 6.89e-04
|
|||||||||
| STKc_ULK1 | cd14202 | Catalytic domain of the Serine/Threonine kinase, Unc-51-like kinase 1; STKs catalyze the ... |
811-929 | 1.47e-03 | |||||
Catalytic domain of the Serine/Threonine kinase, Unc-51-like kinase 1; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. The ATG1/ULK complex is conserved from yeast to humans and it plays a critical role in the initiation of autophagy, the intracellular system that leads to the lysosomal degradation of cellular components and their recycling into basic metabolic units. ULK1 is required for efficient amino acid starvation-induced autophagy and mitochondrial clearance. It associates with three autophagy-related proteins (Atg13, FIP200 amd Atg101) to form the ULK1 complex. All fours proteins are essential for autophagosome formation. ULK1 is regulated by both mammalian target-of rapamycin complex 1 (mTORC1) and AMP-activated protein kinase (AMPK). mTORC1 negatively regulates the ULK1 complex in a nutrient-dependent manner while AMPK stimulates autophagy by inhibiting mTORC1. ULK1 also plays neuron-specific roles and is involved in non-clathrin-coated endocytosis in growth cones, filopodia extension, neurite extension, and axon branching. The ULK1 subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 271104 [Multi-domain] Cd Length: 267 Bit Score: 41.53 E-value: 1.47e-03
|
|||||||||
| PRK12704 | PRK12704 | phosphodiesterase; Provisional |
383-463 | 2.30e-03 | |||||
phosphodiesterase; Provisional Pssm-ID: 237177 [Multi-domain] Cd Length: 520 Bit Score: 41.69 E-value: 2.30e-03
|
|||||||||
| pk1 | PHA03390 | serine/threonine-protein kinase 1; Provisional |
838-882 | 2.55e-03 | |||||
serine/threonine-protein kinase 1; Provisional Pssm-ID: 223069 [Multi-domain] Cd Length: 267 Bit Score: 41.00 E-value: 2.55e-03
|
|||||||||
| DUF4670 | pfam15709 | Domain of unknown function (DUF4670); This family of proteins is found in eukaryotes. Proteins ... |
373-492 | 2.57e-03 | |||||
Domain of unknown function (DUF4670); This family of proteins is found in eukaryotes. Proteins in this family are typically between 373 and 763 amino acids in length. Pssm-ID: 464815 [Multi-domain] Cd Length: 522 Bit Score: 41.48 E-value: 2.57e-03
|
|||||||||
| STKc_STK33 | cd14097 | Catalytic domain of Serine/Threonine Kinase 33; STKs catalyze the transfer of the ... |
844-916 | 3.44e-03 | |||||
Catalytic domain of Serine/Threonine Kinase 33; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. STK33 is highly expressed in the testis and is present in low levels in most tissues. It may be involved in spermatogenesis and organ ontogenesis. It interacts with and phosphorylates vimentin and may be involved in regulating intermediate filament cytoskeletal dynamics. Its role in promoting the cell viability of KRAS-dependent cancer cells is under debate; some studies have found STK33 to promote cancer cell viability, while other studies have found it to be non-essential. KRAS is the most commonly mutated human oncogene, thus, studies on the role of STK33 in KRAS mutant cancer cells are important. The STK33 subfamily is part of a larger superfamily that includes the catalytic domains of other STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 270999 [Multi-domain] Cd Length: 266 Bit Score: 40.61 E-value: 3.44e-03
|
|||||||||
| STKc_SHIK | cd13974 | Catalytic domain of the Serine/Threonine kinase, SINK-homologous inhibitory kinase; STKs ... |
832-895 | 4.12e-03 | |||||
Catalytic domain of the Serine/Threonine kinase, SINK-homologous inhibitory kinase; STKs catalyze the transfer of the gamma-phosphoryl group from ATP to serine/threonine residues on protein substrates. SHIK, also referred to as STK40 or LYK4, is a cytoplasmic and nuclear protein that is involved in the negative regulation of NF-kappaB- and p53-mediated transcription. It was identified as a protein related to SINK, a p65-interacting protein that inhibits p65 phosphorylation by the catalytic subunit of PKA, thereby inhibiting transcriptional competence of NF-kappaB. The SHIK subfamily is part of a larger superfamily that includes the catalytic domains of other protein STKs, protein tyrosine kinases, RIO kinases, aminoglycoside phosphotransferase, choline kinase, and phosphoinositide 3-kinase. Pssm-ID: 270876 [Multi-domain] Cd Length: 290 Bit Score: 40.47 E-value: 4.12e-03
|
|||||||||
| Bud32 | COG3642 | tRNA A-37 threonylcarbamoyl transferase component Bud32 [Translation, ribosomal structure and ... |
826-920 | 8.30e-03 | |||||
tRNA A-37 threonylcarbamoyl transferase component Bud32 [Translation, ribosomal structure and biogenesis]; tRNA A-37 threonylcarbamoyl transferase component Bud32 is part of the Pathway/BioSystem: tRNA modification Pssm-ID: 442859 [Multi-domain] Cd Length: 159 Bit Score: 38.02 E-value: 8.30e-03
|
|||||||||
| Blast search parameters | ||||
|