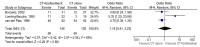Narrative summary
An RCT (crossover, multicentre) conducted in the UK75 (2007) [EL=1+] assessed the efficacy and safety of polyethylene glycol 3350 plus electrolytes (PEG+E) for the treatment of chronic constipation in children. The study included 51 children (29 girls) aged 24 months to 11 years with chronic constipation for at least 3 months. Children were randomised to receive PEG+E (6.9 g powder/sachet) or placebo (6.9 g powder/sachet) for 2 weeks (period I) followed by a 2-week washout period and then a second 2-week treatment period (period II) in which each group received the second medication (period III). The dosing regimen for PEG+E and placebo for children aged 2 to 6 years was: 1 sachet/day on days 1 and 2, 2 sachets/day taken together on days 3 and 4, 3 sachets/day (2 morning, 1 evening) on days 5 and 6 (and 4 sachets/day (2 morning, 2 evening) on days 7 and 8. For children aged 7 to 11 years the dosing regimen was: 2 sachets/day taken together on days 1 and 2, 2 sachets/day taken together on days 3 and 4, 5 sachets/day (2 in the morning, 3 in the evening) on days 5 and 6 and 6 sachets/day (3 in the morning, 3 in the evening) on days 7 and 8. The dosage was adjusted over the first week of treatment in periods I and III and could be adjusted in the second week of each treatment period to determine a dose at which symptoms of constipation did not occur. For both groups, if diarrhoea was present doses were decreased by two sachets or parents were instructed to miss a day of medication. If there were loose stools doses were decreased by one sachet. Safety was monitored by recording adverse events, physical examination findings and weight changes.
There were 31 adverse events among children taking PEG+E (63%) and 28 in children taking placebo (57%) during periods I and III. Most adverse effects were judged to be moderate or mild in severity. Twenty children (41%) on PEG+E experienced 41 events and 22 children (45%) on placebo experienced 45 events judged by the investigator to be at least possibly related to the study treatment. Most of these events were gastro-intestinal disorders, particularly abdominal pain (39 events [39%] in children on PEG+E and 41 events [45%] in children on placebo). One child in the placebo/PEG+E group (the group taking the placebo in the first treatment period) withdrew from the study at week 3 because of abdominal pain, assessed by the investigator as being related to study treatment. This child was taking placebo at the time of withdrawal. New clinically significant abnormalities on physical examination (mainly associated with faecal loading) were found in 13 children (8 out of 27 in the PEG+E/placebo group, 5 out of 24 in the placebo/PEG+E group). When analysed for what these children were taking for the 2 weeks before the physical examination, 23 out of the 24 reports (95.8%) occurred when the child was taking placebo. Only one report of an abnormal abdominal examination occurred while the patient was on PEG+E. The mean weight was similar before and after treatment and no significant difference was found between the two groups for change in weight while on treatment.
An RCT conducted in France66 (2005) [EL=1+] assessed the safety of a PEG 4000 laxative without additional salts in paediatric patients. The study included 96 children (51 male) aged 6 months to 3 years with constipation. Children were randomised to receive either PEG 4000 (non-branded, starting dose: 1 sachet [4 g] and 1 placebo to be taken at breakfast) or lactulose (starting dose: 1 sachet (3.33 g) and 1 placebo to be taken at breakfast) for 3 months. For both drugs, the dose could be doubled if it was ineffective in children aged 13 months to 3 years. If the maximum authorised dose was unsuccessful, one micro-enema of glycerol per day could be prescribed for a maximum of 3 consecutive days. If the child did not produced stools after treatment, 2 enemas could be administered at a 48 hour interval. This procedure was only allowed twice during the study. If the child produced liquid stools for more than 1 day or more than 2 or 3 stools per day depending on age, the dose could be decreased by 1 pair of sachets/day to a minimum of 1 pair of sachets every other day and possibly to transitory interruption. Stool frequency, abdominal pain, vomiting and nausea were recorded by parents on a self-diary evaluation booklet. Assessments were conducted at day 42 (D42) and day 84 (D84) after starting treatment.
Six non serious adverse effects occurred during the study period (5 episodes of diarrhoea in two children in both treatment groups and anorexia in one child on lactulose). Flatulence (either new onset or worsened) lasted significantly longer in children taking lactulose compared to children taking PEG 4000 (PEG 4000: median 3 days, interquartile range 1 to 4.5 days versus lactulose: median 5 days, interquartile range 3 to 19.5 days; P = 0.005). Vomiting episodes (either new onset or worsened) lasted significantly longer in children taking lactulose compared to children taking PEG 4000 (PEG 4000: median 1 day, interquartile range 1 to 2 days versus lactulose: median 2 days, interquartile range 1 to 6 days; P < 0.05). Anal irritation was reported in 5% of the children (2 out of 40, both on lactulose).
There were no differences between PEG 4000 and lactulose groups with regard to other digestive tolerance outcomes. Body height and body weight were unaffected during the 3-month treatment for boys and girls. There were no significant differences between treatment groups for the percentage of children with out of normal range values on D84 compared to baseline status. No treatment-related changes were found in serum iron, electrolytes, total protein, albumin, vitamins A and D and folates. There were no significant differences in the doses used for both medications in either babies or toddlers (babies, PEG: median 1 sachet/day, interquartile range 0.9 to 1 versus lactulose: median 1 sachet/day, interquartile range 1 to 1.3; and toddlers, PEG: median 1 sachet/day, interquartile range 1 to 1.3 versus lactulose: median 1.1 sachet/day, interquartile range 0.9 to 1.5). Treatment stopped in one child in the lactulose group because of lack of efficacy. There were no clinically relevant differences between the two treatment groups at baseline for clinical or biological parameters.
A prospective cohort conducted in the USA69 (2002) [EL=2+] determined the efficiency, acceptability and treatment dosage of polyethylene glycol 3350 without electrolytes during a 12-month treatment period in children with functional constipation and encopresis. The study included 49 children aged 4 years or more referred for functional constipation and encopresis. For 12 months, 28 children (20 boys, mean age 8.7 years ± 3.6, range 4.1 to 17.5 years) received PEG 3350 without electrolytes at an initial dose of 0.5 to 1 g/kg/day and 21 children (17 boys, mean age 7.3 years ± 3.0, range 4.0 to 13.9 years) received magnesium oxide (milk of magnesia [MOM]) at an initial dose of 1 to 2.5 ml/kg. Large laxative dosages could be divided into two daily doses. Parents were told to adjust the dose of medication by 30 ml for PEG 3350 without electrolytes and by 7.5 ml (one-half tablespoon) for MOM every 3 days to a dosage that resulted in one to two soft bowel movements per day and prevented soiling and abdominal pain. If the child retained stools despite compliance with the assigned laxative, daily senna could be added to the treatment. Medication dosage, clinically significant side effects and compliance with medication were assessed at 1, 3, 6, and 12 months after initiating treatment. Patients and parents were provided with diary sheets to record each outcome measured.
At 1 month the mean doses and range for children who were doing well or improved were 0.6 g/kg ± 0.2 (0.3 to 1.1 g/kg) for PEG and 1.4 ml/kg ± 0.6 (0.6 to 2.6 ml/kg) for MOM. At 3 months these were 0.6 g/kg ± 0.3 (0.3 to 1.4 g/kg) for PEG and 1.2 ml/kg ± 0.5 (0.6 to 2.4 ml/kg) for MOM. At 12 months the mean dose of PEG was 0.4 g/kg ± 0.1 (0.1 to 0.7 g/kg). Only two children still required MOM. Their dosages were 0.4 and 1.6 ml/kg, both less than the initial treatment dosage. The mean doses for both treatments at 12 months did not differ significantly between children with or without initial palpable abdominal faecal masses. None of the patients required an increased dosage of either medication over time. Five children received a stimulant laxative in addition to PEG and one child received a stimulant laxative in addition to MOM (P > 0.2). Some children had diarrhoea (number not reported in paper). None of the children in the PEG group became dehydrated. Children receiving PEG and their parents did not report increased flatus, abdominal distension or new onset of abdominal pain. These outcomes were not reported for MOM. No children reported disliking the taste of PEG and no parents reported that their child refused to take it in juice or Kool-Aid. Thirty-three percent of children refused to take MOM.
A retrospective case series conducted in the USA80 (2003) [EL=3] reviewed the efficacy of PEG as a single agent for the treatment of constipation in children with dysfunctional elimination and to assess bladder function following treatment. The study included 46 children diagnosed with dysfunctional voiding and constipation who received polyethylene glycol (PEG) 3350 between January 2000 and July 2002 (35 girls, mean age 7.7 years, range 4.5 to 11.2 years and 11 boys, mean age 7.6 years, range 4.4 to 11.1 years). All children received PEG 3350 without electrolytes at a starting dose of 8 ounces of mixture each day with instructions to adjust the amount consumed by 1 to 2 ounces every 3 days to achieve the goal of one to two soft bowel movements per day. The final dose was normalised according to patient weight and the average final dose was 0.63 g/kg (as reported in abstract) or 0.59 g/kg (as reported in text). The average duration of treatment was 194.3 days (SD 133.5) and side effects were recorded. It is not clear how side effects were measured. Nine of 46 children (all female) reported having diarrhoea. Children with diarrhoea were significantly younger at the start of PEG therapy than children without diarrhoea (patients with diarrhoea (n=9): mean age 6.8 years ± 1.1 versus patients without diarrhoea (n=37): mean age 8.2 years ± 1.8; P = 0.04). The duration of follow-up was significantly longer for children with diarrhoea compared to children without diarrhoea (patients with diarrhoea (n=9): mean 336 days ± 153 versus patients without diarrhoea (n=37): mean 108 days ± 11; P = 0.0028). One child stopped taking PEG because of side effects.
A retrospective case series conducted in the USA81 (2004) [EL=3] evaluated the safety and efficacy of PEG 3350 without electrolytes for the treatment of constipation in children aged under 2 years. The study included 75 children with constipation aged less than 2 years at the start of PEG therapy (mean age 17 months, range 1 to 21 months). Children received PEG 3350 without electrolytes at a starting average dose of 1 g/kg body weight/day. Parents were asked to adjust the dose to yield one to two soft painless stools per day. Adverse effects were measured at 4 months or less (short term, mean 2 months) and 6 months or more (long term, mean 11 months). The average duration of treatment at the short-term assessment was mean 2.3 months ± 1.3, range 1 to 4 months and at the long-term assessment it was mean 10.6 months ± 8.1, range 6 to 37 months. It is not completely clear how side effects were measured, but it seems that parents were asked about them at the time of consultation. At 4 months or less, five children (7%) had experienced ‘runny stools’. The mean dose of PEG used was 1.1 g/kg body weight/day ± 1.2 (median 0.82, range 0.4 to 2.3). At 6 months or more, one child had experienced watery stools. The diarrhoea disappeared after lowering the dose of PEG. The mean dose of PEG used was 0.8 g/kg body weight/day ± 0.4 (median 0.67, range 0.3 to 2.1). Parents did not report increased flatus, abdominal distension, vomiting or new onset abdominal pain. None of the children stopped PEG because of adverse effects. Complete blood counts (in 24 children), electrolytes (in 9 children), renal functions (in 8 children) and liver functions (in 8 children) were occasionally done in children on long-term PEG treatment and all were within normal limits.
A retrospective case series conducted in the USA82 (2004) [EL=3] determined safety, efficacy and optimal dose of PEG powder for treatment of constipation in patients younger than 18 months. The case series included 28 children younger than 18 months treated for constipation with PEG powder. Children received PEG 3350**** at an initial dose of 0.88 g/kg/day (range 0.26–2.14 g/kg/day). After initial dose, families were asked to titrate the dose to obtain at least one non-formed bowel movement daily. Change in dose was permitted within 24 hours, if necessary. The mean duration of treatment was 6.2 months ± 5 (range 3 weeks to 21 months). Children were assessed at an initial visit and subsequent visits every 8 to 12 weeks. The duration of therapy and side effects were retrieved from the patient's chart and the information not available in the chart was obtained by telephone interview. It is not clear how side effects were measured in the first place. The mean effective maintenance dose was 0.78 g/kg/day (range 0.26–1.26 g/kg/day). Side effects were recorded in 17.9% of patients. One infant (3.6%) experienced increased passage of gas per rectum, whereas four infants (14.3%) experienced transient diarrhoea that resolved after dose adjustment.
A prospective case series conducted in the USA83 (2003) [EL=3] assessed the biochemical and clinical safety profile of long-term PEG 3350 treatment in a large cohort of children and also its acceptance by paediatric patients. The study included 83 children older than 2 years (48 males, 35 females, mean age 7.4 years, range 2.0 to 16.9 years) with chronic constipation who were treated daily with PEG for more than 3 months. For an average of 8.7 months (range 3–30 months) all children received PEG 3350 without electrolytes orally at an initial dose of 0.8 g/kg/day. Parents were asked to adjust dose of PEG solution as required to yield two soft painless stools per day. Over time, parents were instructed to gradually decrease the dose of PEG if the symptoms of constipation and encopresis showed improvement. Adverse effects, both clinical and laboratory variables, were assessed. Parents were interviewed using a structured questionnaire and asked about any possible adverse effects of PEG and particularly about excessively loose or frequent stools, abdominal pain, flatulence, bloating and nausea. Following interview and physical examination, 4 ml of blood was obtained for measurement of different parameters.
Clinical adverse effects were minor and over the mean duration of therapy. Eight patients (10%) experienced frequent watery stools some time during therapy, but diarrhoea disappeared with reduction of the dose. Five children (6%) experienced bloating or flatulence and two children (2%) abdominal pain. Different individual patients (1%) experienced each of the following: thirst, fatigue and nausea after receiving PEG solution on an empty stomach. General physical examination findings revealed no new significant abnormalities compared with the pre-treatment. None of the patients stopped treatment due to adverse effects and all were to continue PEG therapy.
Laboratory evaluation results (haemoglobin, haematocrit, serum electrolytes, blood urea nitrogen, serum creatinine, serum albumin and osmolality) were normal in all patients (10 patients did not have serum osmolality measured). Ten patients (11%) had slightly elevated alanine transaminase (ALT) level (less than 1.5 times the upper limit of normal, range 31 to 45 units per litre [U/L]). Eight of these patients had ALT levels re-measured within 8 weeks, seven of whom were still receiving PEG therapy. Seven of these eight patients had values in the reference range, one had a slightly elevated ALT level (less than 1.2 times normal, 28 U/L). Three patients (4%) had an elevated aspartate aminotransferase level (less than 1.5 times normal, range 42-52 U/L) and all had normal values when re-measured while still receiving PEG therapy. Both the dose and the duration of PEG therapy were not significantly different in patients with abnormal values compared with those with laboratory values in the reference range.
A prospective case series conducted in Australia84 (2007) [EL=3] evaluated the safety and efficacy of a PEG 3350-based preparation containing electrolytes in the treatment of chronic constipation in children. The study included 77 children with chronic constipation for at least 6 months, which was either untreated or inadequately treated by laxatives (44% boys, mean age 4.9 ± 2.6 years). Children received PEG 3350 plus electrolytes for an average of 75.5 days. Starting dose (number of sachets/day) during the first 5 days was established according to children's age (children aged 2 to 6 years: 1 sachet/day on days 1 and 2, 1 sachet twice a day on days 3 and 4, 1 sachet three times a day on day 5; children aged 7 to 11 years: 1 sachet twice a day on days 1 and 2, 2 sachets twice a day on days 3, 4 and 5). Thereafter, and until end of the study, the dosage was titrated according to the faecal form. This dose was increased by 1 sachet/day in the event of continued hard stools or no bowel movements, and decreased by 1 to 2 sachets/day in the event of loose stools or diarrhoea. Adverse effects were monitored throughout the study: blood samples for laboratory investigation were taken at baseline, 28 days and 84 days after initiating treatment. Vital signs were measured at baseline and 84 days after initiating treatment. It is not clear how other clinical adverse effects were collected.
The mean numbers of sachets/day during the treatment period was 1.3 (6.9 g). Seventy-two children (92%) reported a total of 318 adverse events. Two hundred and forty-one (76%) of those events were assessed as unrelated to the study treatment, 262 (82%) were considered mild and 302 (95%) had resolved by the end of the study. Six serious adverse events occurred in four children: four affected the gastrointestinal system while the other two were not clearly reported. All of them were assessed by the investigator as unrelated or unlikely to be related to the study medication and were resolved at the end of the study. One serious adverse event (faecal impaction) led to one patient's premature withdrawal from study as the child was admitted to hospital for bowel washout. No clinically significant changes in vital signs as a result of the study medication were observed.
A prospective case series conducted in Sweden85 (2005) [EL=3] assessed the effectiveness of PEG 3350+E over the course of long-term treatment in children with constipation. The case series included 134 children referred with constipation and/or encopresis (88 males, age not clearly reported). All children received PEG 3350+E (13.8 g sachets) at a mean starting dose of 0.58 sachets for children aged 2 to 6 years and 0.51 sachets for children aged 7 to 11 years. Doses were adjusted in each patient to achieve symptom relief with the minimally effective dosage. The mean duration of treatment was 50 weeks (SD ±50 weeks, range 1 to 211 weeks). The final treatment dose and side effects were recorded but it is unclear how this was done. The mean dose at the end of the observational period was 0.42 sachets for children aged 2 to 6 years and 0.49 sachets for children aged 7 to 11 years. The overall mean change was 0.553 to 0.477 sachets per day. Side effects were reported in 10 patients (7.5%) and these were reported as generally mild and transient.
An RCT conducted in the USA70 (2006) [EL=1-] compared the efficacy, safety and patient acceptance of PEG 3350 without added electrolytes versus magnesium oxide (milk of magnesia, MOM) over 12 months. The study included 79 children diagnosed with functional constipation with faecal incontinence (65 boys, age range 4 to 16.2 years, median age 7.4 years, mean 8.1 ± 3.0). Children were randomised to receive PEG 3350 without added electrolytes at 0.7 g/kg body weight daily for 12 months or MOM 2 ml/kg body weight daily for 12 months. If it was necessary children were disimpacted with one or two phosphate enemas in the clinic on the day of the visit and then started laxative therapy that evening. Outcomes were patients' acceptance and adherence. Patients and their parents were questioned with respect to side effects during each visit.
Several children complained about the taste of both PEG and MOM. Two children (5%) continued to refuse PEG versus 14 children (35%) who continued to refuse MOM during the 12 months of the study (P < 0.001). By 12 months 27 children (34%) had left the study or were lost to follow-up. In the PEG group, two children were lost to follow-up monitoring, two (5%) had refused PEG, one child was allergic to PEG and two children were receiving senna. These seven children were counted as not improved and not recovered. In the MOM group two children were lost to follow-up, three children had discontinued study participation, 14 children (35%) had refused to take MOM and one child was receiving senna. Mean treatment doses at 1 month were 0.7 ± 0.2 g/kg body weight for PEG and 1.2 ± 0.7 ml/kg body weight for MOM. At 3 months doses were 0.6 ± 0.3 g/kg body weight for PEG and 1.2 ± 0.8 ml/kg body weight for MOM. Mean treatment doses were similar in children who improved and those who did not improve for both treatments. There were no other significant clinical effects for either medication, apart from transient diarrhoea disappearing with dose reduction.
A retrospective cohort conducted in the USA86 (2003) [EL=2-] reported efficacy of PEG therapy, effective dose and patient compliance separately for children with constipation and children with constipation and encopresis over the long term. This included 74 children (40 boys) aged over 2 years with chronic constipation treated daily with PEG 3350 without electrolytes for more than 3 months. Children received PEG 3350 without electrolytes at a starting dose of 0.8 g/kg/day. Parents were asked to adjust the dose as required to yield two soft painless stools per day. The average duration of the treatment was 8.4 months (range 3 to 30 months) and adverse effects were assessed. Some outcomes variables on effectiveness were gathered by interviewing patients and/or parents and examining patients, but it is unclear how data on adverse effects were obtained. The average dose of PEG at the time of evaluation was 0.73 g/kg/day (range 0.3 to 1.8) following adjustment of dose by the carers. No major clinical adverse effects were observed.
A prospective case series conducted in the USA87 (1987) [EL=3] prospectively monitored children receiving large doses of mineral oil throughout the early phase of treatment. The study included 25 children with constipation, aged over 1 year with no previous treatment with mineral oil (mean age 7.83 years, range 1.75 to 14.27 years). Following initial disimpaction children received 45 ml mineral oil twice daily between meals for a period of 4 months. The dose was gradually decreased on a monthly basis (usually 30 ml/month) depending on the patient's reported performance and the results of serial rectal examinations (month 1: mean dose 4.0 ± 1.4 (SEM), month 2: mean dose 2.9 ± 1.2, month 3: mean dose 2.1 ± 0.5, month 4: mean dose 1.4 ± 0.4). Serum beta-carotene levels, retinol levels and alfa tocopherol levels were measured at baseline and at the end of every treatment month.
Mean retinol levels at 1 and 2 months were not significantly different from baseline values. After 3 months levels significantly increased compared to baseline (baseline: 1.48 micromols/l ± 0.84 SEM (42.3 micrograms/dl ± 24.1), treatment: 2.22 micromols/l ± 0.77 (63.5 micrograms/dl ± 22.1); P < 0.01) but changes were not significant after 4 months. Mean serum beta-carotene levels decreased significantly at 1 month, 2 months and 3 months when compared to baseline, but there were no significant differences after 4 months:
month 1 (n=25): baseline: 1.0 micromols/l ± 0.5 SEM, (55.7 micrograms/dl ± 26.0) versus treatment: 0.7 micromols/l ± 0.4, (35.9 micrograms/dl ± 22.1); P < 0.01
month 2 (n=17): baseline: 1.1 micromols/l ± 0.6, (59.5 micrograms/dl ± 30.6) versus treatment: 0.7 micromols/l ± 0.5, (38.2 micrograms/dl ± 28.4); P < 0.05
month 3 (n=10): baseline: 1.1 micromols/l ± 0.6 (60.4 micrograms/dl ± 30.0), treatment: 0.6 micromols/l ± 0.2, (34.7 micrograms/dl ± 12.3); P < 0.05.
Serum alfa tocopherol levels remained relatively unchanged throughout the study. No statistical significant difference was found between baseline levels and those obtained throughout the 4 months of therapy.
An RCT (crossover) conducted in the UK71 (1977) [EL=1-] compared effectiveness and side effects of a standardised senna syrup with lactulose in the treatment of childhood constipation. The study included 21 children aged under 15 years with a history of constipation treated at home for 3 months or more. Children were randomised to receive either senna syrup (10 to 20 ml daily) for 2 weeks or lactulose (10 to 15 ml daily) for 2 weeks with 1 intermediate week with no treatment. Each preparation was given throughout the appropriate treatment week in a daily dose varied according to the age of the patient. Outcome measures were stool consistency, number of stools passed each day and adverse effects. These outcomes were recorded by parents in written diaries.
There were significantly more adverse effects (number of patients) during the senna week (12 including eight colic, one diarrhoea, two colic plus diarrhoea, one colic plus distension) compared to the lactulose week (one colic) (P < 0.001). There were no significant differences between the week with no treatment (four including three colic and one colic plus distension) compared to the lactulose week (one colic). One patient on senna at the beginning of study failed to attend at the end of the first week assessment but was included in the analysis.
An RCT conducted in Iran72 (2007) [EL=1-] compared the clinical efficacy and safety of liquid paraffin and lactulose in the treatment of functional childhood constipation. The study included 247 children with chronic functional constipation aged 2 to 12 years (mean age 4.1± 2.1 years). All children received one or two enemas daily for 2 days to clear any rectal impaction (30 cc/10 kg of paraffin oil). Children were randomised to receive either liquid paraffin orally (n=127) 1 to 2 ml/kg twice daily for 8 weeks or lactulose orally (n=120), 1 to 2 ml/kg twice daily for 8 weeks. For determination of the best dose for each child, parents were asked to increase the volume of each drug by 25% every 3 days as required to yield one or two firm to loose stools. Outcome measures were optimal dose of drug and side effects. Parents received a chart to record side effects.
The final mean effective dose was significantly larger in children taking lactulose compared to children taking liquid paraffin (2.08 ml/kg/day ± 0.21 versus 1.72 ml/kg/day ± 0.13; P < 0.001). Apart from nausea and hard stool, side effects during weeks 4 to 12 were more frequent***** in children taking liquid paraffin compared to children taking lactulose: abdominal pain (50 versus 10), bad palatability (40 versus 15), pain at defecation (50 versus 10), bloating (20 versus 10), diarrhoea (30 versus 10), anal oil leakage (40 versus 20), flatulence (20 versus 10), nausea (5 versus 10) and hard stool (6 versus 20). No children in either group experienced vomiting.
An RCT conducted in Turkey73 (2005) [EL=1-] compared the efficacy, safety and optimal dose of liquid paraffin and lactulose in children with chronic functional constipation. The study included 40 children aged 2 to 12 years referred for evaluation of constipation with evidence of faecal impaction (22 male, mean age 3.7 years ± 2.7). Children were randomised to receive either liquid paraffin or lactulose for 8 weeks. The medication was administered orally as a suspension at 1 ml/kg twice daily for each drug. To determine the best dose for each child, parents were asked to increase or decrease the volume of each drug by 25% every 3 days as required to yield two firm to loose stools per day. The maximum dose used throughout the study was 3 ml/kg per day for each drug. Outcomes measured at 4 and 8 weeks after initiation of treatment were: optimal dose of drugs, compliance rate and side effects. Patients were instructed to take both empty and full containers to calculate the amount of medication taken. It is unclear how side effects were recorded.
The optimal dose of drugs was not significantly different for children taking liquid paraffin compared to children taking lactulose (mean 1.88 ml/kg/day ± 0.27 versus mean 2.08 ml/kg/day ± 0.27). These data were reported in a table and it was assumed that figures given were for the whole study period. Data reported in text for the last 4 weeks of treatment stated the optimal dose for liquid paraffin as 1.72 ml/kg/day ± 0.18 and for lactulose as 1.82 ml/kg/day ± 0.57. Adherence rate during the first 4 weeks of treatment was not significantly different when comparing both groups. During the last 4 weeks of therapy significantly more children complied with taking liquid paraffin than the children taking lactulose (n=90 versus n=60; P = 0.02). No patient stopped treatment because of adverse effects (adverse effects not reported). During the first 4 weeks, taste aversion was reported in one child on liquid paraffin and abdominal distension in two patients on lactulose influenced adherence. During the last 4 weeks, adherence was influenced by poor symptom control in five patients, side effects (abdominal distension and cramping) in three children on lactulose and watery stools in two children on liquid paraffin.